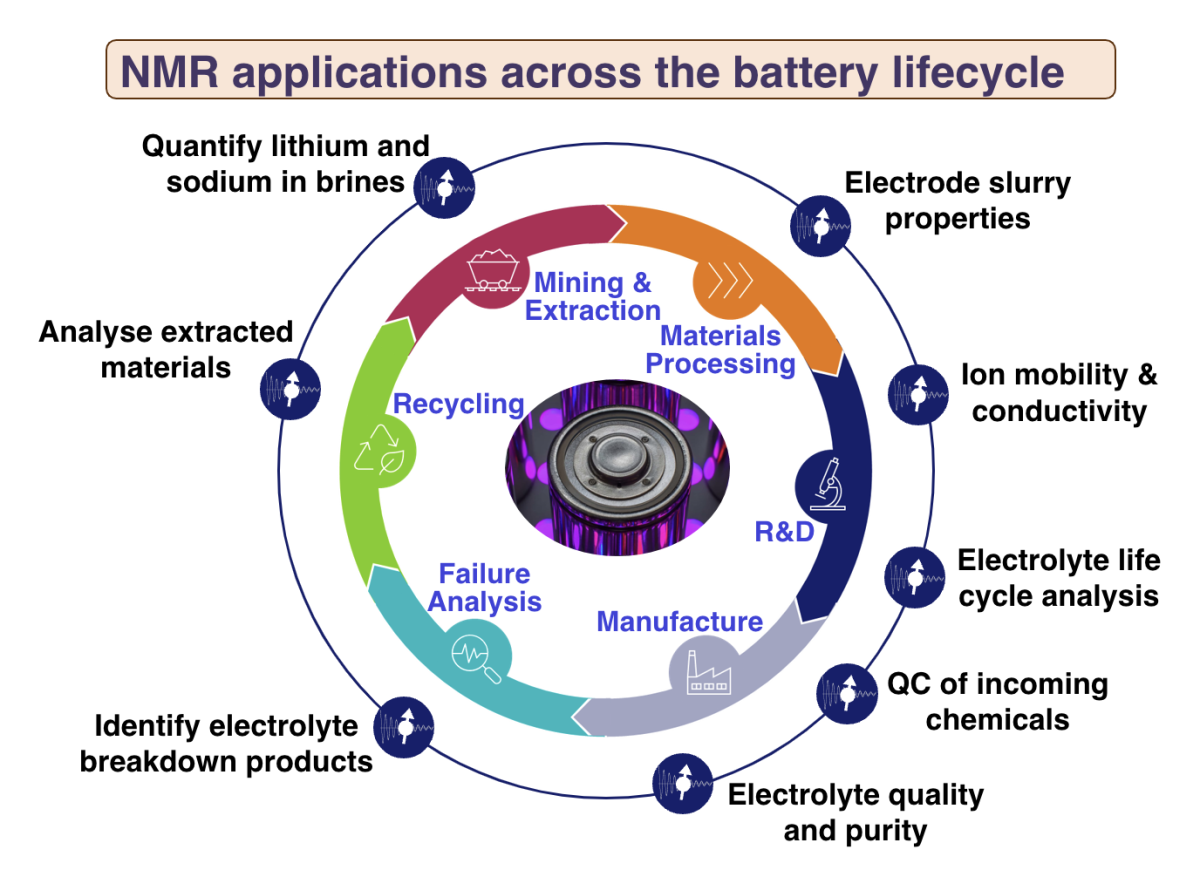
Products
Applications
Learning
The demand for more effective, more sustainable energy storage requires accelerated development of new battery technologies and optimising the manufacturing of current products. Benchtop NMR provides critical materials characterisation for both the current carrying liquid electrolytes and battery systems across the full battery lifecycle. The compact X-Pulse broadband benchtop NMR brings the core analytical capabilities of NMR spectroscopy to the different environments required for battery R&D and QC. Adding broadband multi-nuclei capability enables characterisation of all the key chemical species in electrolytes within a single instrument and significantly extends the applications.

Currently, most commercial batteries expected to come into production in the next 5 years use liquid state electrolytes. This makes NMR a perfect analytical tool for supporting their development. The electrolyte transfers positively charged ions between the battery electrodes causing charging and discharging and provides a free deintercalation environment for ions.
Electrolytes contain a wide variety of salts, organic solvents and additives. Electrolyte compositions can have significant differences in thermal resistance, chemical stability, ionic conductivity, and electrode compatibility. These parameters greatly affect performance, lifetime, safety, and the scope of the battery’s application. Therefore, comprehensively characterizing the electrolyte in combination with understanding and controlling its method of action are indispensable in development and quality control of battery technology.
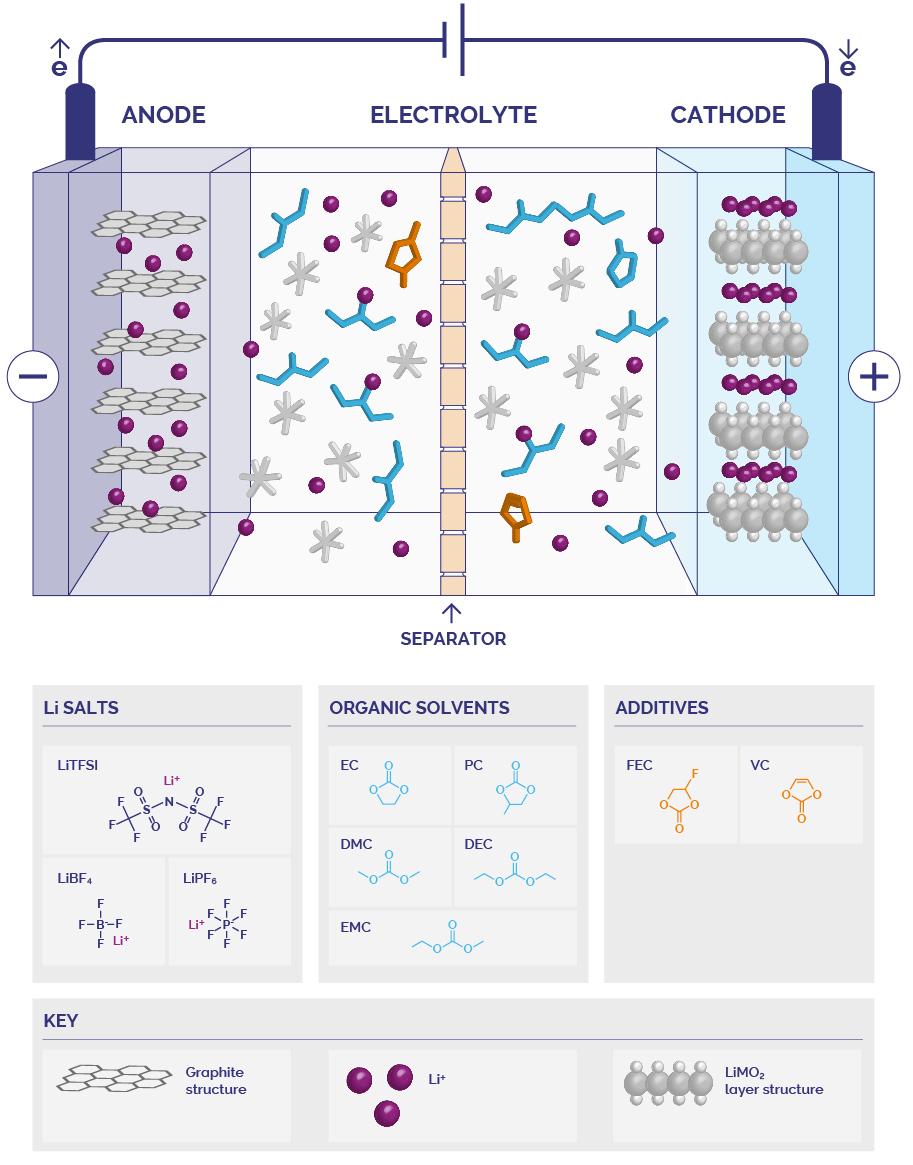
Benchtop NMR accelerates R&D of electrolyte formulations providing quantitative insights into the drivers of higher energy/power density, longer life span and lower cost. Current electrolytes contain Li+ or Na+ salts dissolved in a mixture of organic solvents, typically ethyl carbonate (EC), diethyl carbonate (DEC), dimethyl carbonate (DMC) or propylene carbonate (PC). Understanding their diffusion properties is key to improving the performance of new formulations. Using our X-Pulse broadband benchtop spectrometer, the diffusion coefficient, ionic conductivity, and transference number of all the different electrolyte species can be measured quickly and in the same lab in which the developmental chemistry takes place. The full range of NMR active nuclei available in this single broadband X-Pulse instrument means that your cation (⁷L, ²³Na), anion (³¹P, ¹¹B, ¹⁹F) and solvent (¹H,¹³C) components can all be analysed independently.
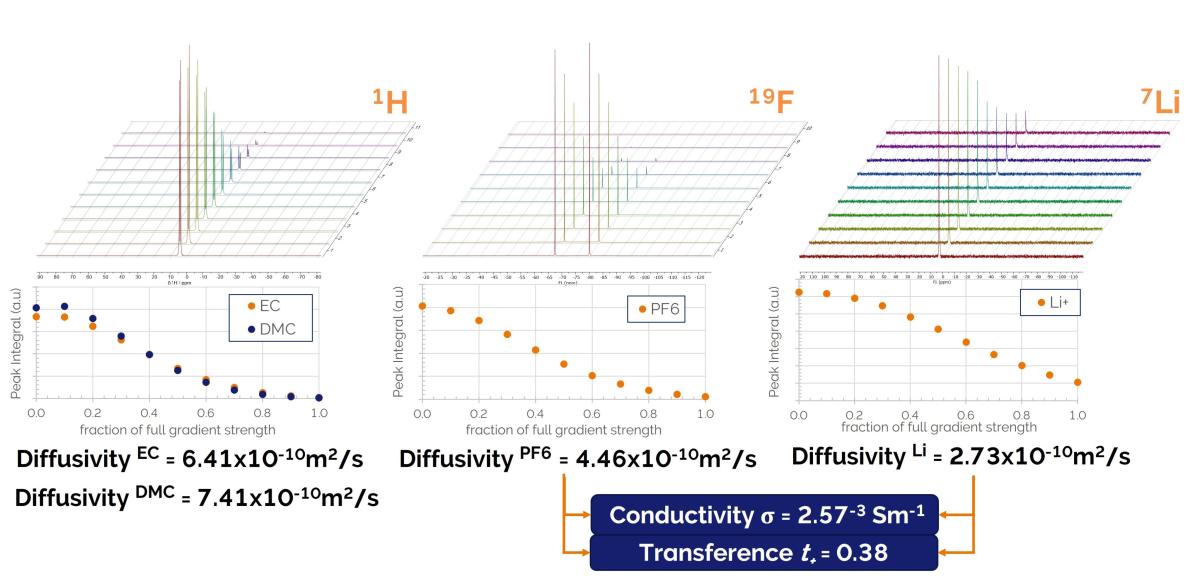
Diffusion measurements for a lithium battery electrolyte solvent mixture. Pulsed Field Gradient Spin Echo (PFGSE) experiments
The exact compositions of chemicals, solvents, additives, and trace impurities are determined with Quantitative NMR (qNMR) to help optimise electrolyte viscosity, permittivity, and long-term chemical stability. For example, adding carefully controlled quantified concentrations of trace polymer based impurities can promote advantageous insoluble film formation at the solid electrolyte interphase (SEI). These films decrease the battery self-discharge rate and improve the coulombic efficiency.
Broadband benchtop NMR provides incoming raw materials, and final product checking of the chemical composition and purity of these complex mixtures including:
Quantitative NMR (qNMR) additionally determines the precise concentrations of chemicals, solvents, additives, and trace impurities in the final product. The areas reported under the NMR signal of each species being directly proportional to its concentration.
App Note: qNMR Optimises Battery Electrolytes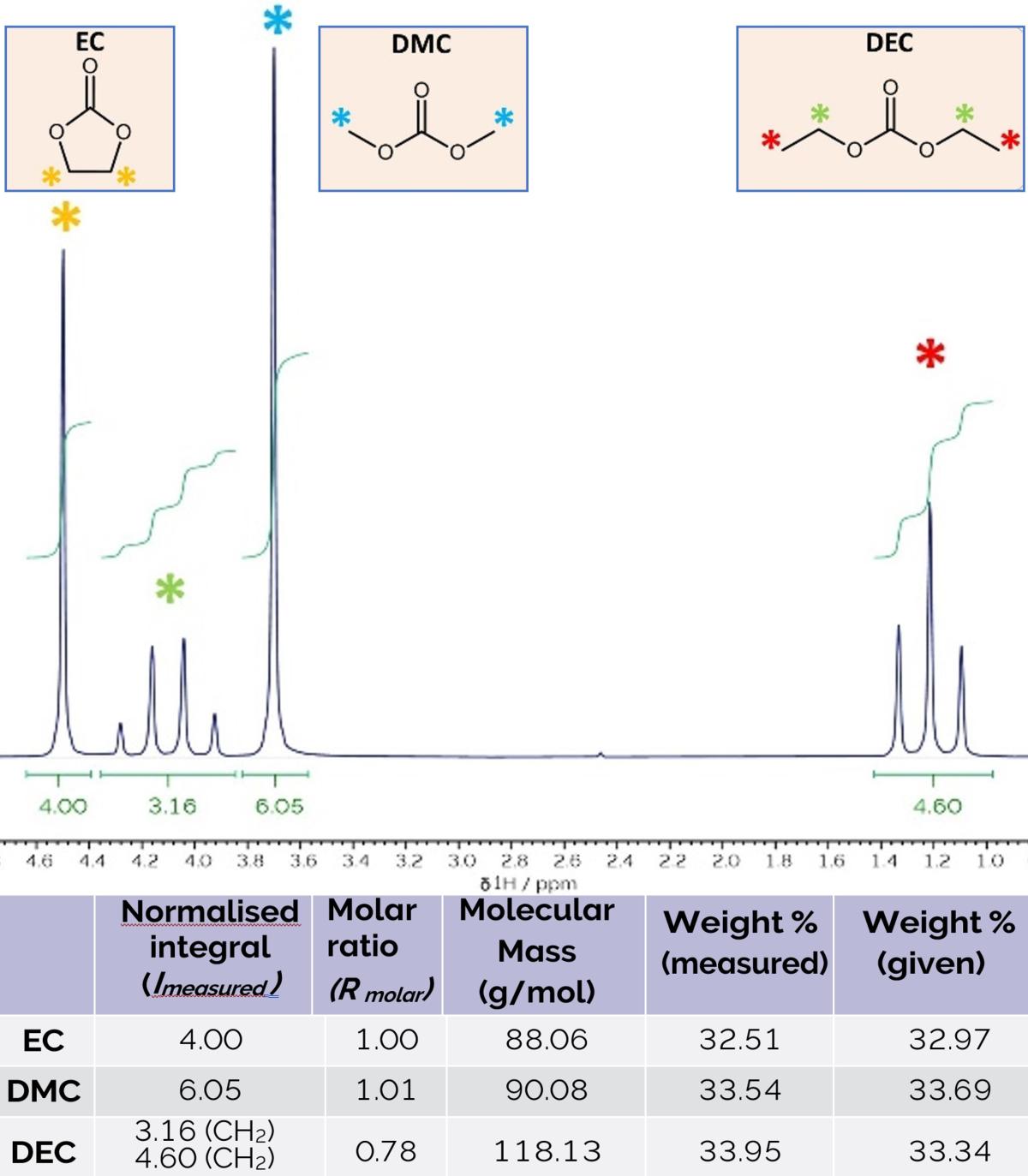
1H-NMR spectrum of a mixture of ethylene carbonate, dimethyl carbonate, and diethyl carbonate with their molar and mass ratios determined from qNMR
During and following electrolyte degradation, breakdown products are quickly identified and the source of in-cell performance differences in commercial batteries can be diagnosed. NMR reaction monitoring determines the competing reaction kinetics to identify causes and mechanisms of degradation. Additionally, pulsed field gradient NMR quantifies differences in physical properties including self-diffusion of “good” and degraded electrolytes.
Most electrolyte breakdown products that can form during battery charge and discharge cycles are identified from a single 19F NMR spectrum. The flexibility of benchtop NMR provides the capability of rapid electrolyte checks after each discharge cycle to pinpoint the processes causing degradation.
App Note: Monitoring Battery Electrolyte Decomposition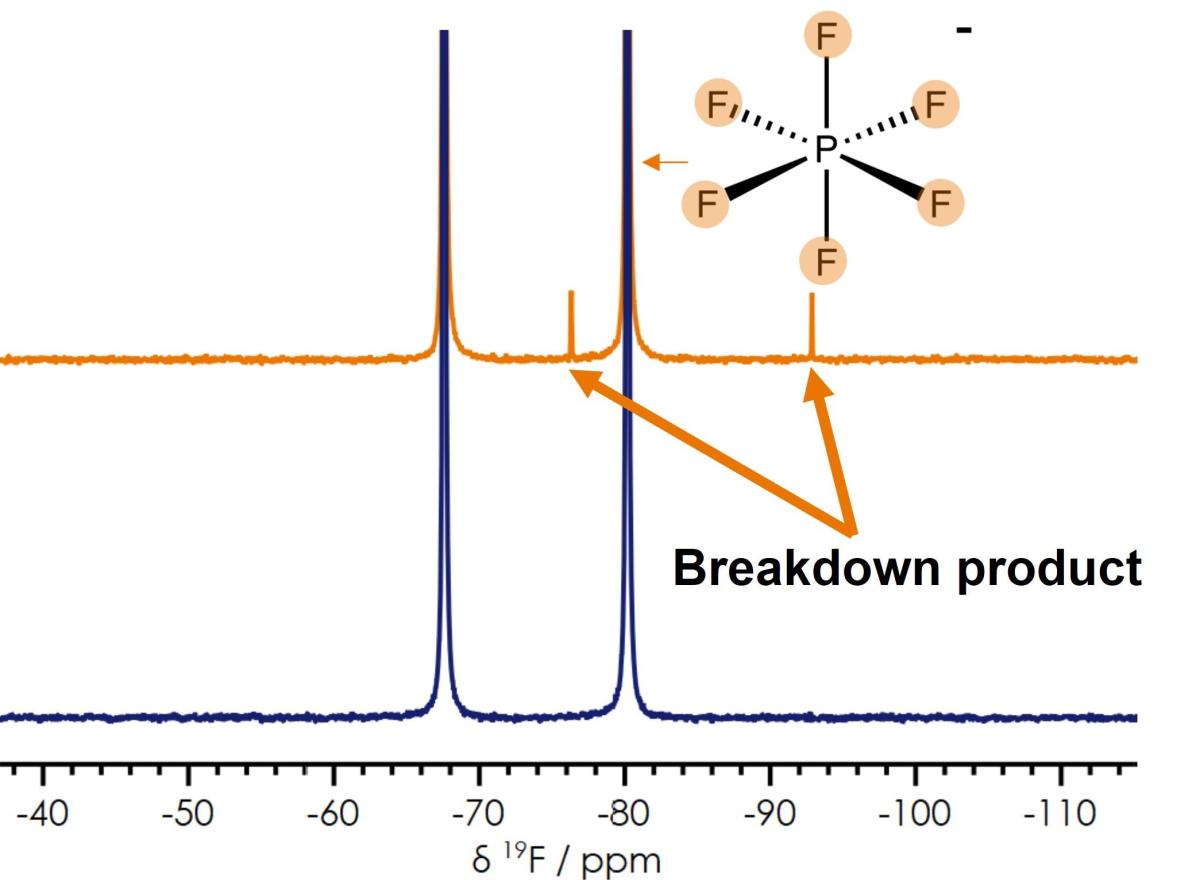
Electrolyte breakdown products (Difluorophosphoric acid) from the presence of unwanted water) identified from the Fluorine NMR spectrum
Benchtop NMR can provide end to end lifecycle characterisation from initial raw materials mining right through to circular economy recycling to recover valuable materials or explore second-life options: