Products
Applications
Learning
Benchtop Nuclear Magnetic Resonance (NMR), sometimes called compact NMR, puts a powerful analytical technique at your fingertips. NMR spectrometers are widely used for the structural and compositional analysis of materials, from small molecule fine chemicals to polymers, across almost every industry. Benchtop NMR spectrometers democratise that applicability by enabling analyses to be conducted in a typical laboratory or factory manufacturing environment. There are 2 types of benchtop NMR:

TD-NMR system (MQC+) for quality control of fat in food | Benchtop NMR spectrometer (X-Pulse) with autosampler
Most people think of NMR instruments as large steel canisters filled with liquid cryogens taking up entire rooms. This is because of the large static magnetic field typically generated by a cooled superconducting magnet.
In contrast, benchtop NMR is cryogen-free. The large static magnetic field is instead generated using a rare earth permanent magnet. This reduces the footprint of NMR instruments and significantly extends the locations and environments where it is used. It does, however, mean that a lower total static field (often referred to as B0) is achieved:- typically in the range of 0.5 – 2.5 tesla (T). Usually field is specified in terms of the 1H frequency at that field: approximately 20 – 100 megahertz (MHz)*. This is easily sufficient for a wide range of chemical, physio-chemical, and materials analysis. Although the resolution is lower for spectroscopy compared to high field NMR, benchtop NMRs are more versatile, simpler to use and require minimal maintenance. The capability to operate these compact systems in new environments provides some completely novel applications. For example, placing an NMR system next to your reaction vessel and flowing reaction mixture through it, enables real time reaction monitoring, including reaction dynamics characterisation.
Talk to our experts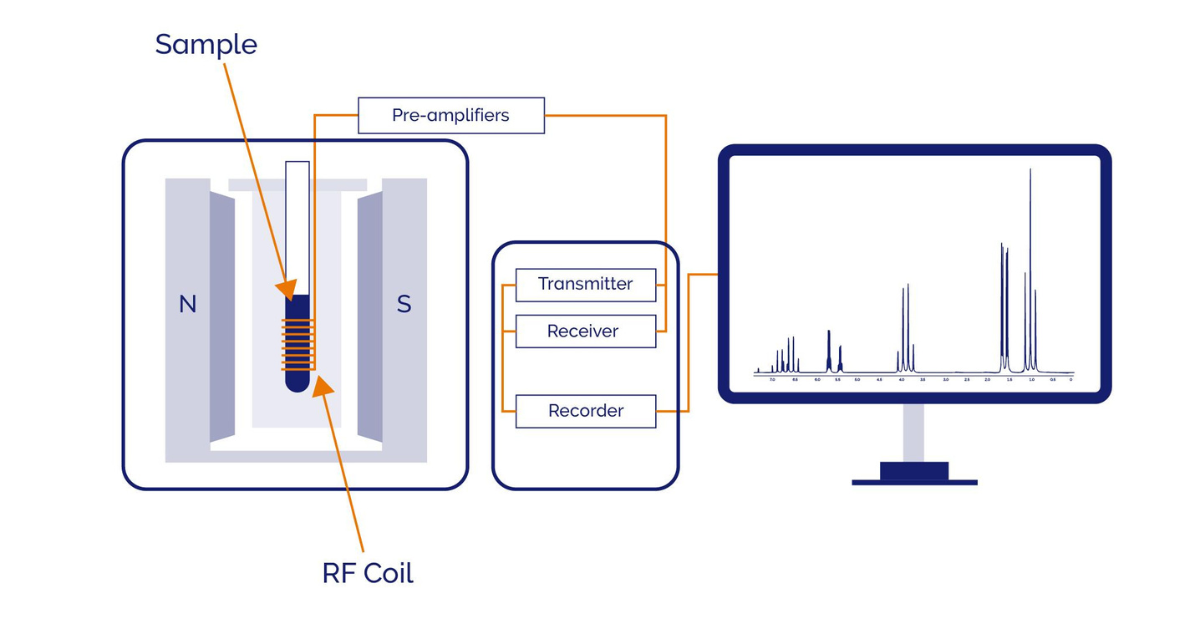
Schematic of a typical NMR system
NMR seems on the surface seems quite simple. Although the underlying physics and mathematics can become complex; for many applications a top level understanding of “why” you get different signals is sufficient. The NMR signal comes from the nucleus of an atom, hence “nuclear”. Many nuclei have a quantum mechanical property called spin, that is non-zero. This makes that nucleus act like a tiny bar magnet. In a real sample, you have many of those tiny bar magnets. When they are placed in a large magnetic field, a substantial proportion of those nuclei align parallel to this external magnetic field at any given time. The number of them aligning parallel or antiparallel depends on:
With benchtop NMR, the number is typically less than 10 spins per million. Once the spins are aligned, one or more radio frequency pulses are applied and excite the aligned spin. As those spin relax from the excited state to the initial state, they precess or resonate.
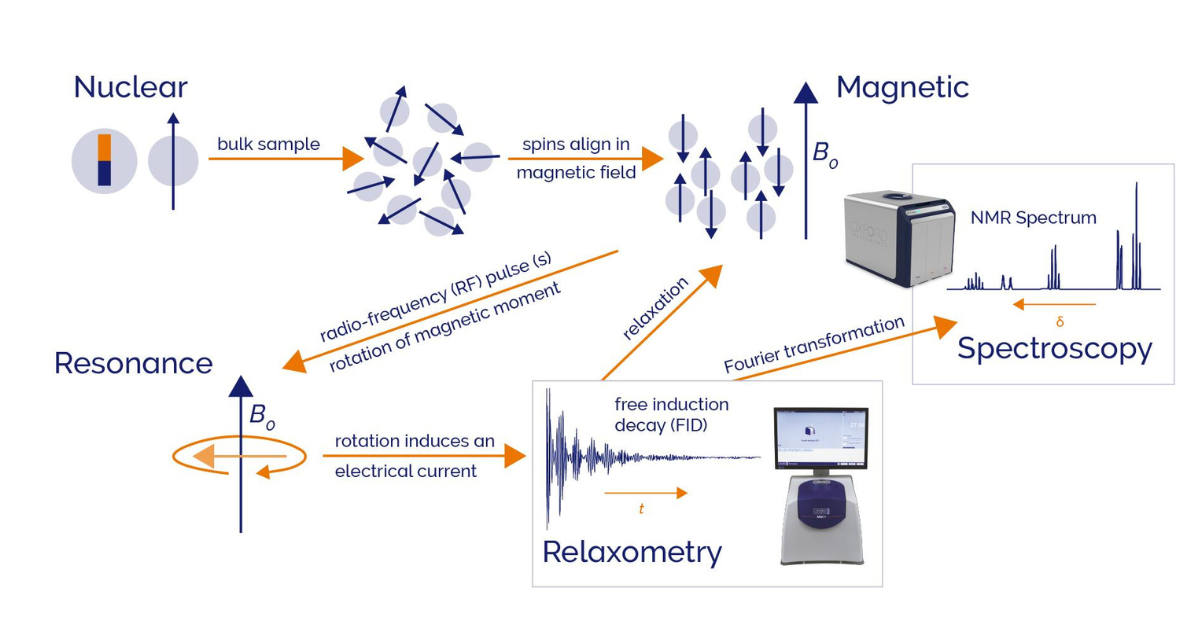
Visual depiction of NMR signal generation.
The movement of these tiny magnets induces a voltage in the detection electronics, which is the raw NMR signal. In its simplest form, this is called the free induction decay, FID. This cycle of excitation and relaxation can be repeated to accumulate signal and reduce noise. This relaxation data is used widely in time domain NMR (TD-NMR) which is also referred to as NMR relaxometry. It provides physiochemical data particularly for quantifying the amount of a particular compound. NMR is inherently quantitative as the amount of signal is directly proportional to the number of nuclei in the sample. For instance, TD-NMR quantifies fat content in foods, oil and moisture content in seeds, fluorine content in polymers and the oil pick up of coatings in textile manufacturing. Operators require minimal training and are often unaware the automated pass/fail analysers they use are in fact NMR instruments!
People more often think of NMR as a spectroscopy method. For this, the raw NMR signal is Fourier transformed from signal as a function of time to signal as a function of frequency to generate the commonly known NMR spectrum. This NMR spectrum provides a wealth of chemical and structural information: from the structure and chemical composition of specific compounds and quantifying their amounts in mixtures, to the type of chemical environments present in a molecule.
Most benchtop NMR systems, whether time domain or spectroscopy, are fully featured enabling users to additionally carry out a much wider range of experiments. A few examples are:
NMR signals from solids have very short decay times, which are difficult to detect. Measurements are therefore made usually on liquids within the samples, which have longer-lasting, easier to detect, signals.
In TD-NMR, although the samples are usually solids (e.g. seeds or food samples), it is the liquid content and amount inside the sample that is measured. Samples are inserted in tubes typically of diameter 10mm upwards.
In NMR Spectroscopy, 5mm diameter tubes are used that are too small to put a solid sample into. Liquid samples are created, either by dissolving the sample in a suitable solvent or by extracting the liquid for analysis.

TD-NMR sample vials for seed and snack food analysis
In summary, the data from compact benchtop NMR instruments significantly expand the applications the technique can be used for. Importantly, benchtop NMR instruments now enable NMR which was previously a research technique in core laboratories and central facilities to be placed almost anywhere in a lab and positioned at or near line in many manufacturing environments.
*In NMR, frequency is the rate of precession of the magnetic moment of a nuclei’s proton around the applied external magnetic field. The frequency for a 1H nuclei in MHz = γ(42.58)*B0.
Contact our Applications Teams