Products
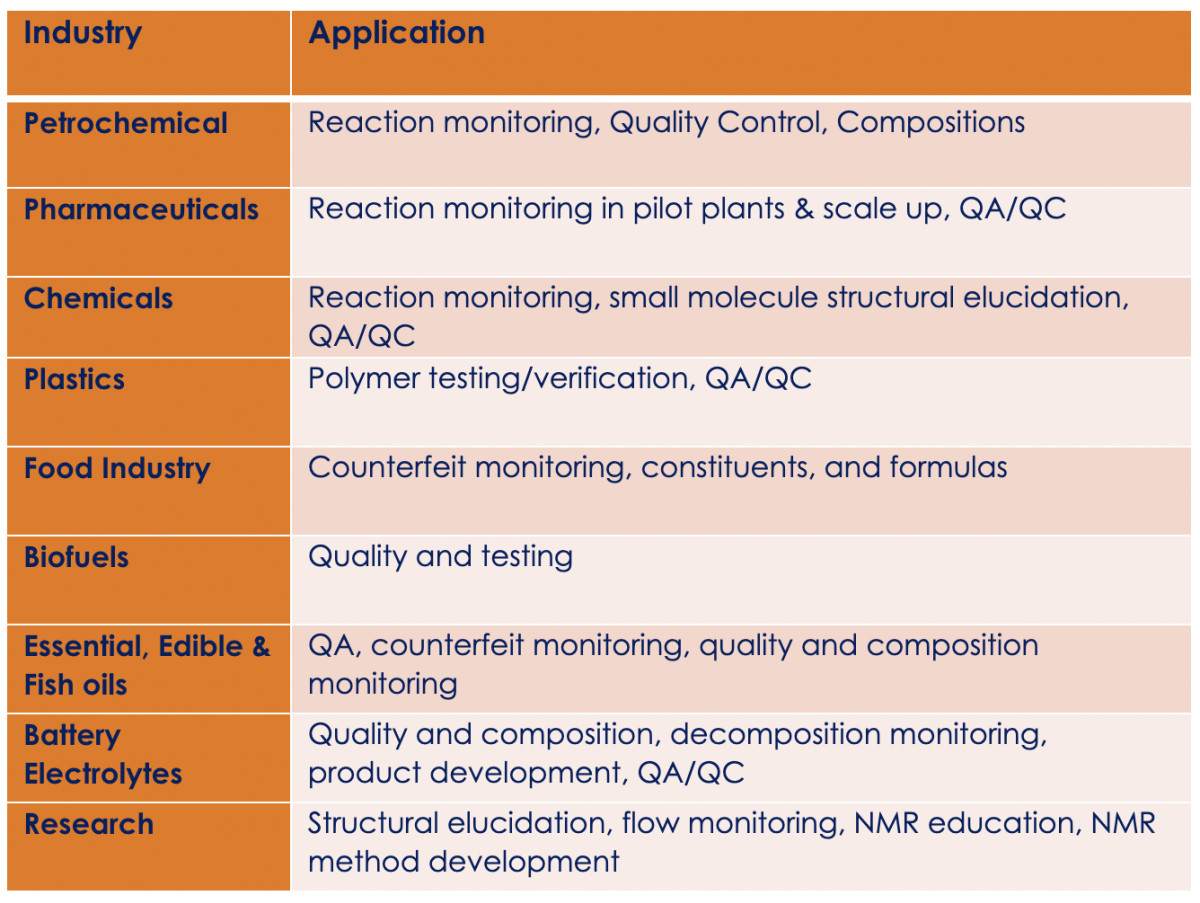
Applications
Learning
Just as with high field NMR, benchtop NMR spectroscopy uses the spectrum produced by an NMR experiment to uncover chemical and structural information about molecules and compounds. Furthermore, the ability to integrate instruments with reaction syntheses, locate them near-line in manufacturing and move them around facilities opens up a host of additional applications. Amongst the information benchtop NMR provides is:
A modern benchtop NMR system, such as the X-Pulse, replaces the bulky and expensive to run superconducting magnet of a high-field instrument, with a cryogen-free rare-earth permanent magnet. This permits the instrument to be significantly more affordable, smaller, and able to be sited in a much wider range of locations.

The main components of benchtop NMR instruments are the permanent magnet which surrounds a cylindrical chamber (known as the probe), plus control electronics and a data station. The sample is placed in the probe under the influence of the magnetic field. Coils around the probe irradiate radiofrequency pulses that cause the chemical nuclei in the sample to resonate at specific frequencies. These coils also detect the emitted NMR signal. As nuclei in molecules interact with the electrons present, a subtle shift occurs in the frequency of the nuclei (measured in parts per million, ppm). This is known as the chemical shift and is consistent across magnetic fields for differing types of functional groups in chemistry. See the below diagram for common chemical shift ranges of hydrogen nuclei and carbon nuclei:
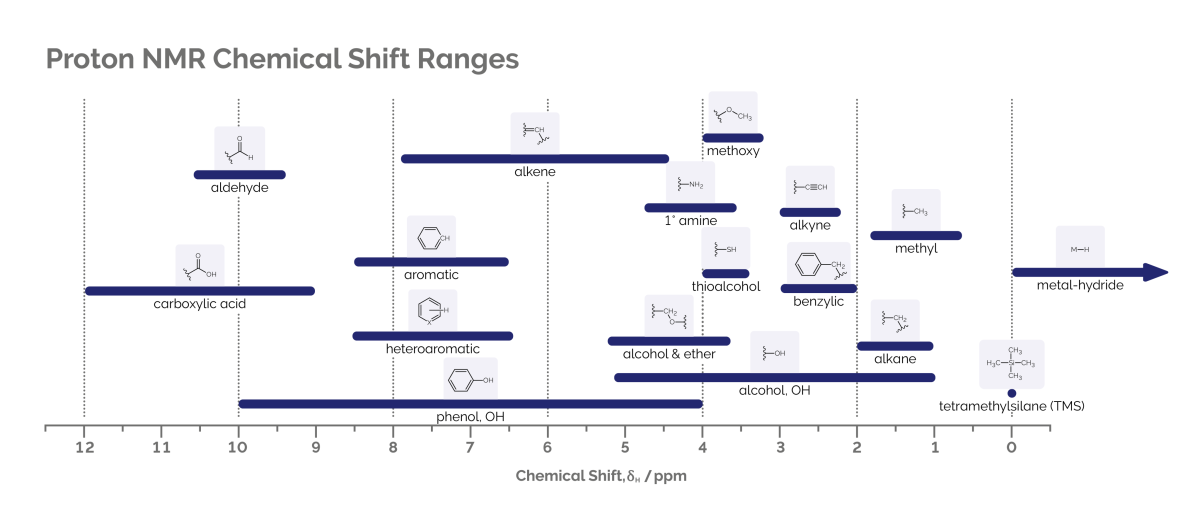
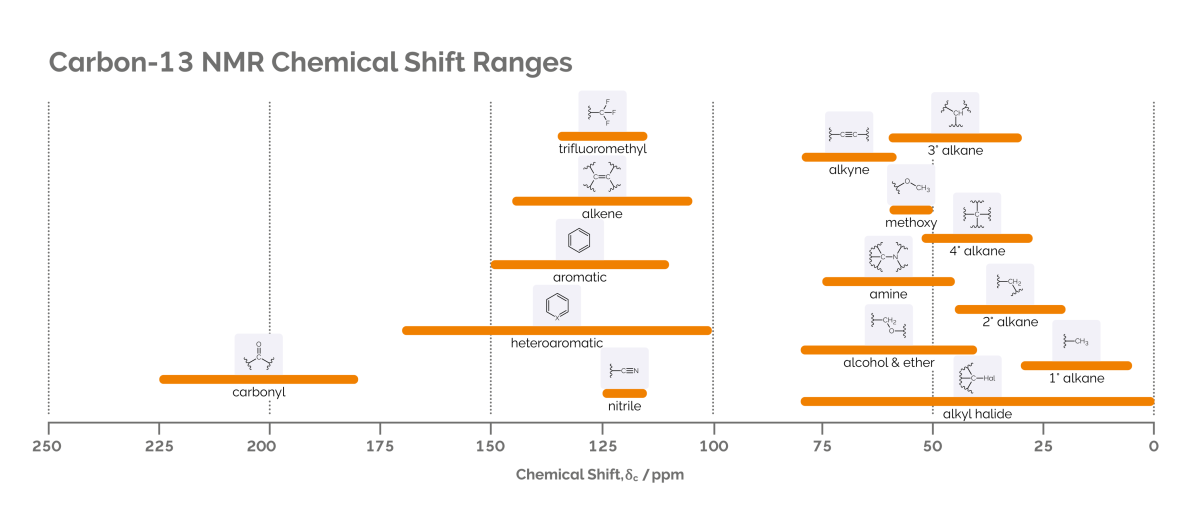
As well as electrons, nuclei in a molecule also interact with other nuclei. This interaction produces a ‘splitting’ of the lines in the spectrum and is known in NMR spectroscopy as ‘J-coupling’. The splitting pattern gives a spectroscopist information about the other nuclei present in the molecule, and their chemical environment. This J-coupling, combined with the chemical shift, gives rise to NMR’s remarkable ability to aid structural elucidation of molecules.
The spectrum below shows how a combination of chemical shift and J-coupling patterns allow the peaks to be fully assigned. The small organic molecule ethyl crotonate has five chemically distinct proton environments observed by 1H NMR spectroscopy where the chemical shift corresponds to the chemical functionality, and the pattern of peaks which make up the signal provide information on connected nuclei.
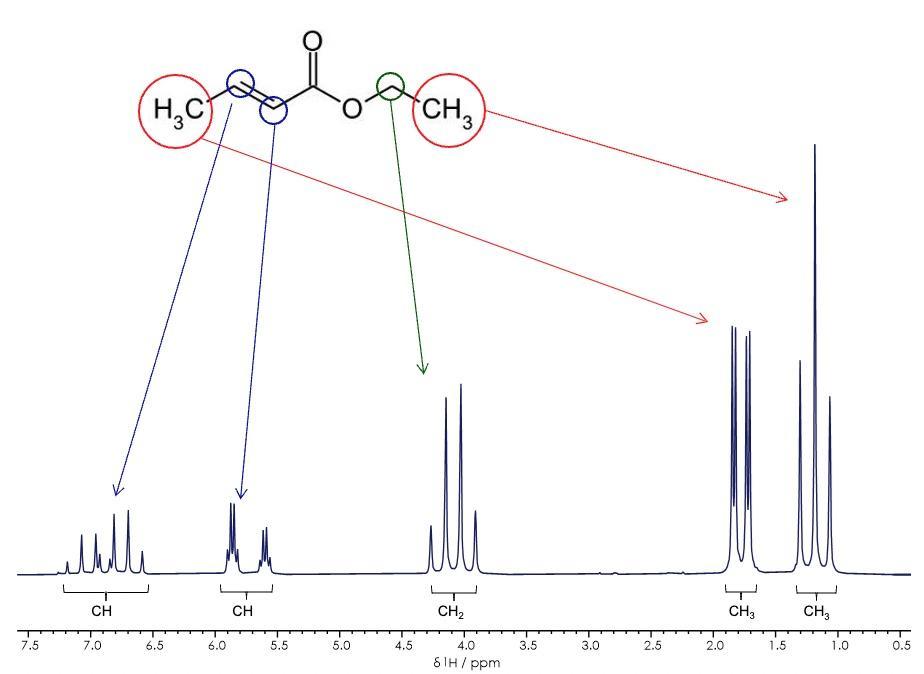
NMR signals are inherently quantitative, so when measured with appropriate parameters an NMR spectrum not only identifies the molecules in the sample, it also tell users about the concentration.
The chemical shift and J-coupling associated with different chemicals means that each spectrum becomes a ‘fingerprint’ for the chemical or mixture of chemicals. This is very powerful in quality assurance and quality control (QA/QC) applications when identifying a loss in performance or a difference in the chemicals being supplied by a 3rd party. This ‘fingerprinting’ has a wide range of other applications, such as coffee authentication, and assisting law enforcement in identifying novel psychoactive substances (NPS).
In addition, benchtop NMR spectroscopy can provide another dimension of analysis by acquiring two-dimensional spectra. As well as the usual chemical shift and coupling patterns found in one-dimensional spectra, these provide information on which nuclei are bonded or correlated with one another. These advanced experiments help enhance structural elucidation experiments as shown in our app note.
In summary, benchtop NMR spectroscopy is a powerful non-invasive, non-destructive analytical technique that brings structural and quantitative data into any laboratory and many manufacturing environments.
Contact our Applications Teams