Products
Applications
Learning
Ensuring consistent potency of drug product is crucial for patient safety and reliable product efficacy. An important application of NMR spectroscopy involves testing drug potency by measuring the amount of API. Generally, two approaches are used for API testing: quantitative NMR (qNMR) and time-domain NMR (TD-NMR).
As well as providing a vital quality check of finished drug products, this type of testing plays an important role in ensuring authenticity and identifying counterfeit drugs.
Time-Domain NMR (TD-NMR) is commonly used in structural analysis. TD-NMR can selectively measure fluorine (19F) in tablets, powders, pastes and solutions “as-is” without the need to us breakup the matrix using harmful chemicals. Therefore, given that a high proportion of APIs contain fluorine, it is possible to quantify the API content in tablets or powders. TD-NMR is already widely used to measure the fluoride content in toothpastes which are classified as drugs, not cosmetics, therefore the level of fluorides must be carefully controlled and measured accurately at different stages of production and product release.
A key attribute of NMR is that the data acquired is inherently quantitative, as the size of any given signal is directly proportional to the number of nuclei producing it. As a result, NMR is an excellent technique for both absolute and relative quantification. Often, frequency domain qNMR techniques use the internal standard approach, using a known amounts of a standard added to a sample for quantification based on the integrated intensities of signals from the standard and the compound of interest. This method is particularly advantageous when the sample being tested is a novel compound, or it is difficult to obtain an appropriate standard of known concentration. Moreover, as both analyte and standard are measured together, this method has the potential to be extremely accurate.
Another method, which can offer greater ease-of-use, and is particularly popular in time domain NMR methods is the standard curve approach. This method is often adopted for QA/QC applications, such as determining the concentration of the active ingredient of a drug in production, the moisture content of a tablet, or the concentration of fluoride in toothpaste. To create a curve, an appropriate standard is selected, the signal is measured at several concentrations, and standard linear regression is used to fit the data. The sample signal is then compared to the calibration, allowing the absolute concentration to be determined with a high degree of accuracy and repeatability. The calibration curve approach is widely used in many time domain NMR applications due to the underlying stability of the instruments ensuring consistent results.
Table 1 shows the results of the measurements of the quantity of APIs for five typical small molecule pharmaceuticals. One-dimensional 1H spectra were collected on high-field and low-field nuclear magnetic resonance spectrometers, and API contents of the five pharmaceutical raw materials quantified, using chloroform as an internal standard. Values obtained for all the analysed drugs were comparable whether obtained using a high field spectrometer or on the benchtop. Moreover, in both the 600 MHz and 60 MHz evaluations, the serial number I drug measurements were approximately half of the manufacturer's claimed value, clearly demonstrating the ability of benchtop NMR to detect an inferior product.
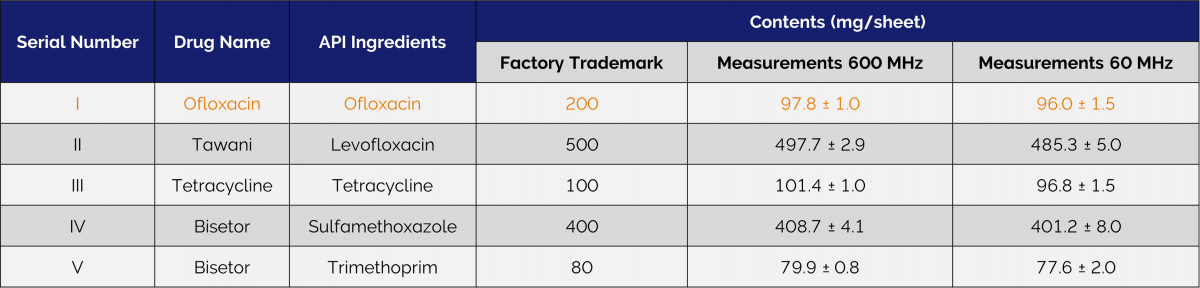
Table 1. API content determination of five pharmaceutical drug formulations
APIs and formulated drug products are required to go through an appropriate amount of stability testing prior to regulatory approval. NMR is used routinely in stability testing as an identification test, and it may also be used to confirm the identity of degradation products and quantify them in the parent material.
It is important to measure moisture content in powders to prevent them from caking during processing. Moisture is also an important parameter affecting the microbiological safety and stability of tablets and lyophilised proteins. TD-NMR can measure moisture in powders and tablets non-destructively.
Quality also extends to the properties of the excipients which affect how APIs are released. TD-NMR can measure the amorphous and crystalline components in both the raw materials and tablets which may affect their dissolution.