Products
Applications
Learning
Pharmaceutical manufacturing must be consistent – to meet regulatory requirements, batches from different sites, or produced at different times, or under different conditions, must all produce the same approved end product. In the quest for consistency and accuracy in pharmaceutical manufacturing, applications for NMR are many and diverse, from raw material testing to purity assessment, reference standard qualification, and determination of impurities or solvent content.
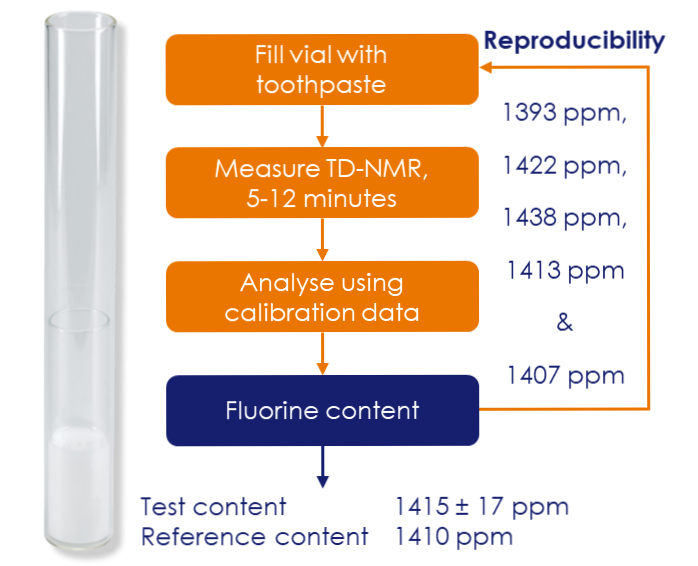
Figure 1: Time domain NMR (TD-NMR) results for repeat measurements of the same adult toothpaste, demonstrating consistent repeatability.
A key attribute of NMR is that the data acquired is inherently quantitative, as the size of any given signal is directly proportional to the number of nuclei producing it. As a result, NMR is an excellent technique for both absolute and relative quantification of any given compound, as demonstrated for duroquinone in Figure 2. Reference standards, including impurity reference standards, require full molecular characterisation, and NMR is commonly used as an identification test with quantitative NMR (qNMR) being used to provide purity data.
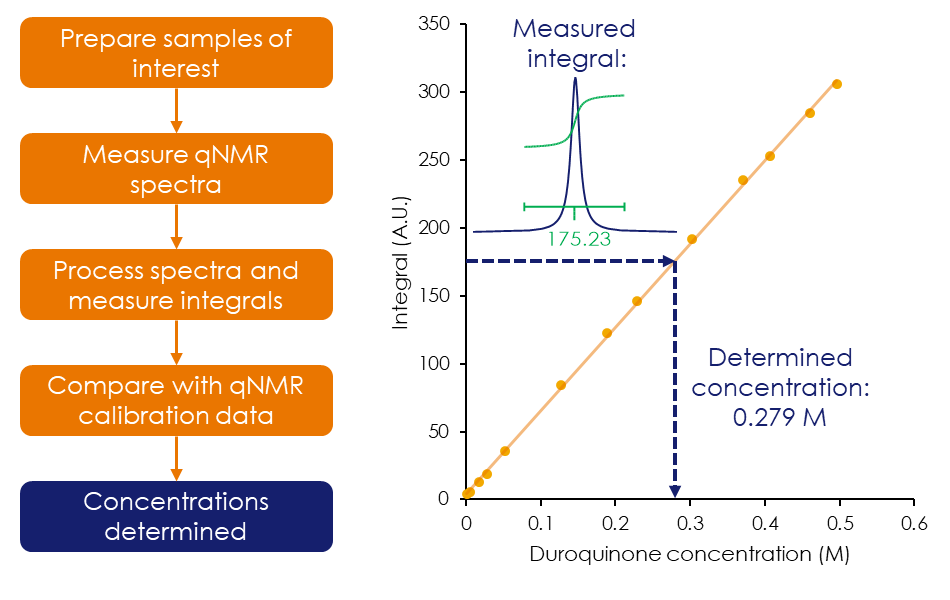
Figure 2: Determining the concentration of duroquinone using an external standard by benchtop qNMR.
Testing of raw materials involves the characterisation of the raw material to ensure that it is as expected, and NMR provides a fast and efficient way to assess these raw materials across multiple batches. Purity is often assessed with a qNMR experiment performed against an external standard. Once these tests have been performed and purity and suitability have been established, the material can be released into the manufacturing process. This testing is necessary to ensure consistent supply and conformity of raw materials, which are often required in large quantities but must be confirmed to be ‘as described’ before use, to ensure that the end product is ‘as required’.
Testing during the drug manufacturing process is key to avoiding plant shut-downs due to sub-standard product. Laboratory tests are usually performed at various stages along the manufacturing route to ensure that intermediates and drug products confirm with reference standards. This testing is performed by the NMR analysis of a sample of material against a quantitative reference standard of known purity, using pre-determined quantitative experimental parameters.
More recently, the inclusion of NMR in flow chemistry for continuous manufacturing has opened the door for even greater efficiencies. As the pharmaceutical industry is making considerable efforts to establish continuous manufacturing as an alternative to batch production, automated quality control processes that ensure all reactions are controlled and monitored throughout the process bring tremendous advantages. Benchtop NMR spectroscopy can provide a solution in this setting, addressing the requirements of automated chemical production with robust, real-time, quality evaluation data.