Products
Applications
Learning
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
The The X-Pulse benchtop broadband multinuclear spectrometer incorporates three channels for the observation of different nuclei: one for a deuterium (hydrogen-2) lock signal; another for the high frequency proton (hydrogen-1) and fluorine-19 signals; and the third, broadband X-channel, easily and quickly tuneable to a wide range of other nuclei (19% - 41% of ¹H frequency, ²⁹Si - ³¹P), often referred to by NMR spectroscopists as X-nuclei. When comparing different nuclei, and their suitability for NMR, there are a number of important considerations. Usually described as spin, abundance, frequency and receptivity. Find out more about how these factors affect NMR spectroscopy in our application note.
In this short video we demonstrate the acquisition of spectra for seven nuclei, on a single X-Pulse; showing how straightforward and fast it is to tune and match the X-Pulse to different nuclei and samples.
Selected NMR active nuclei observed on a single X-Pulse broadband benchtop NMR spectrometer are detailed below:
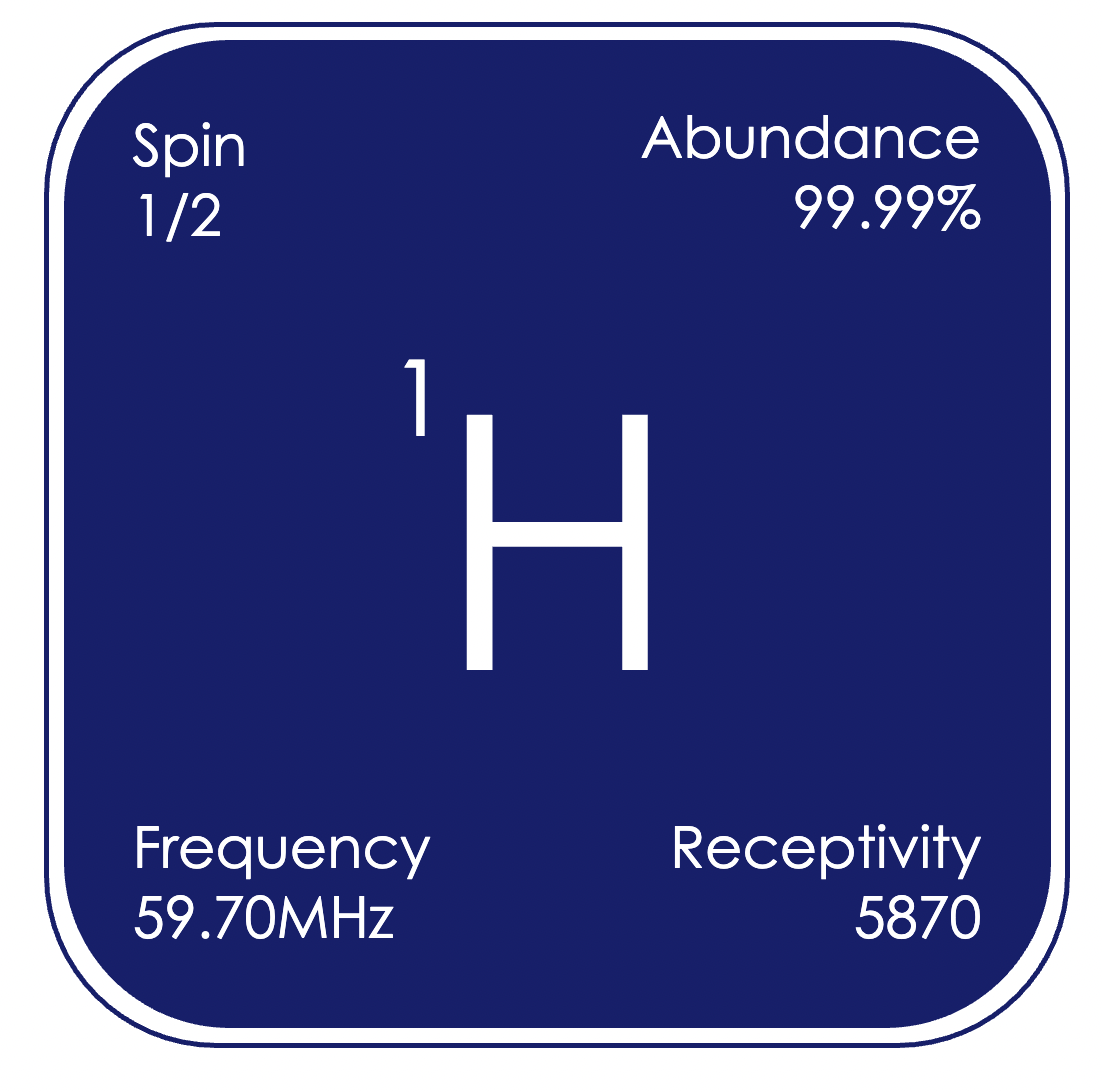
¹H (proton) is the archetypal nucleus used in NMR spectroscopy, with the highest receptivity of any nuclei, and present in the vast majority of chemical compounds. Signals for simple diamagnetic organic and organometallic compounds are observed over the range of δH −5 to +15 ppm (relative to (CH₃)₄Si in CDCl₃), and the correlations between observed chemical shifts and chemical environments are well established.
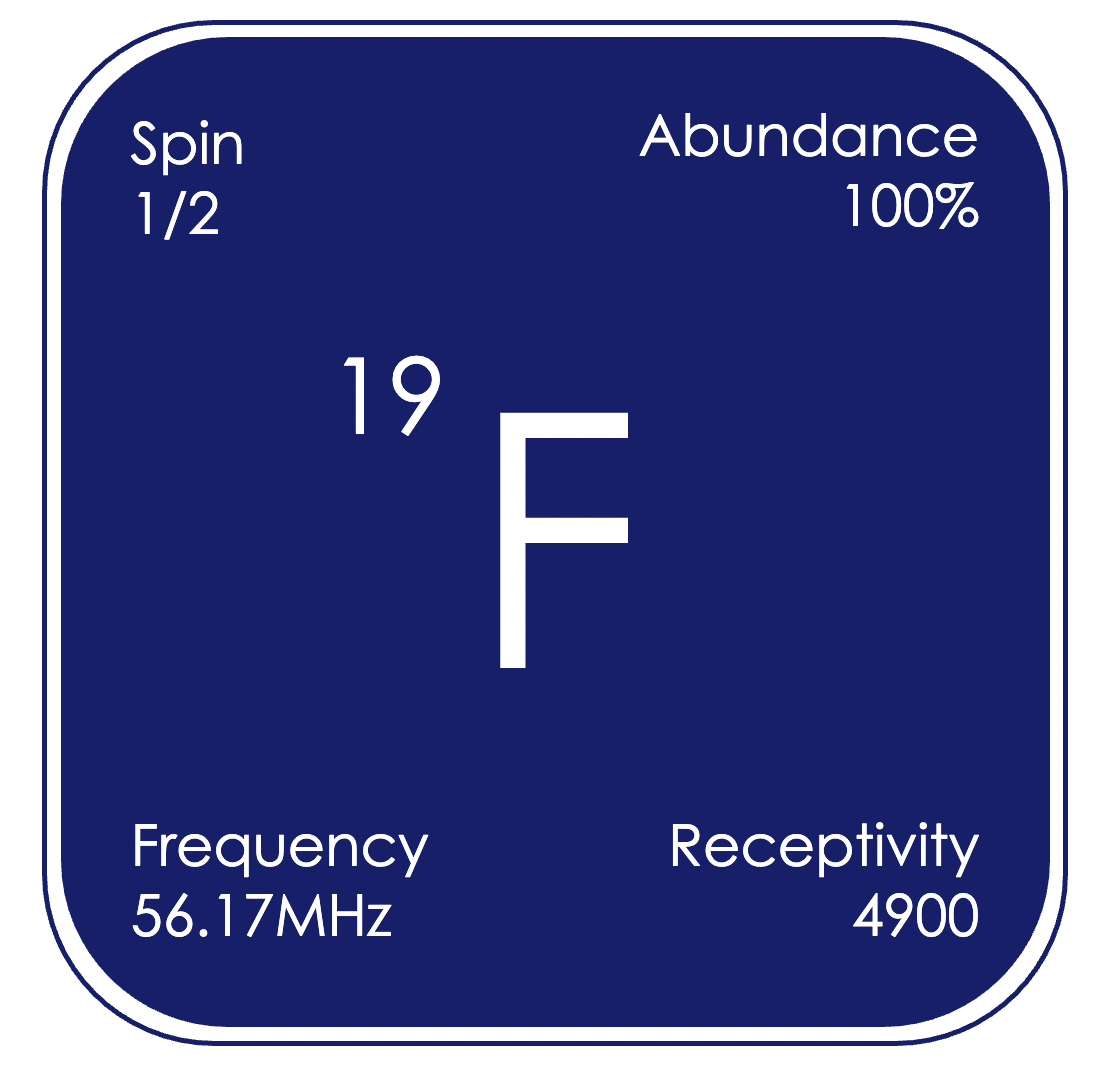
As the second highest receptivity nuclei, ¹⁹F is commonly studied by NMR spectroscopy, and has a much wider spectral window than for proton, of δF +100 to -250 ppm (relative to CCl₃F). Like proton, ¹⁹F is a spin 1/2 nucleus, and fluorine-fluorine, and fluorine-proton couplings are commonly observed. Fluorine containing compounds are used in a huge range of applications; including pharmaceuticals, bulk chemicals, and electrochemical devices such as batteries.
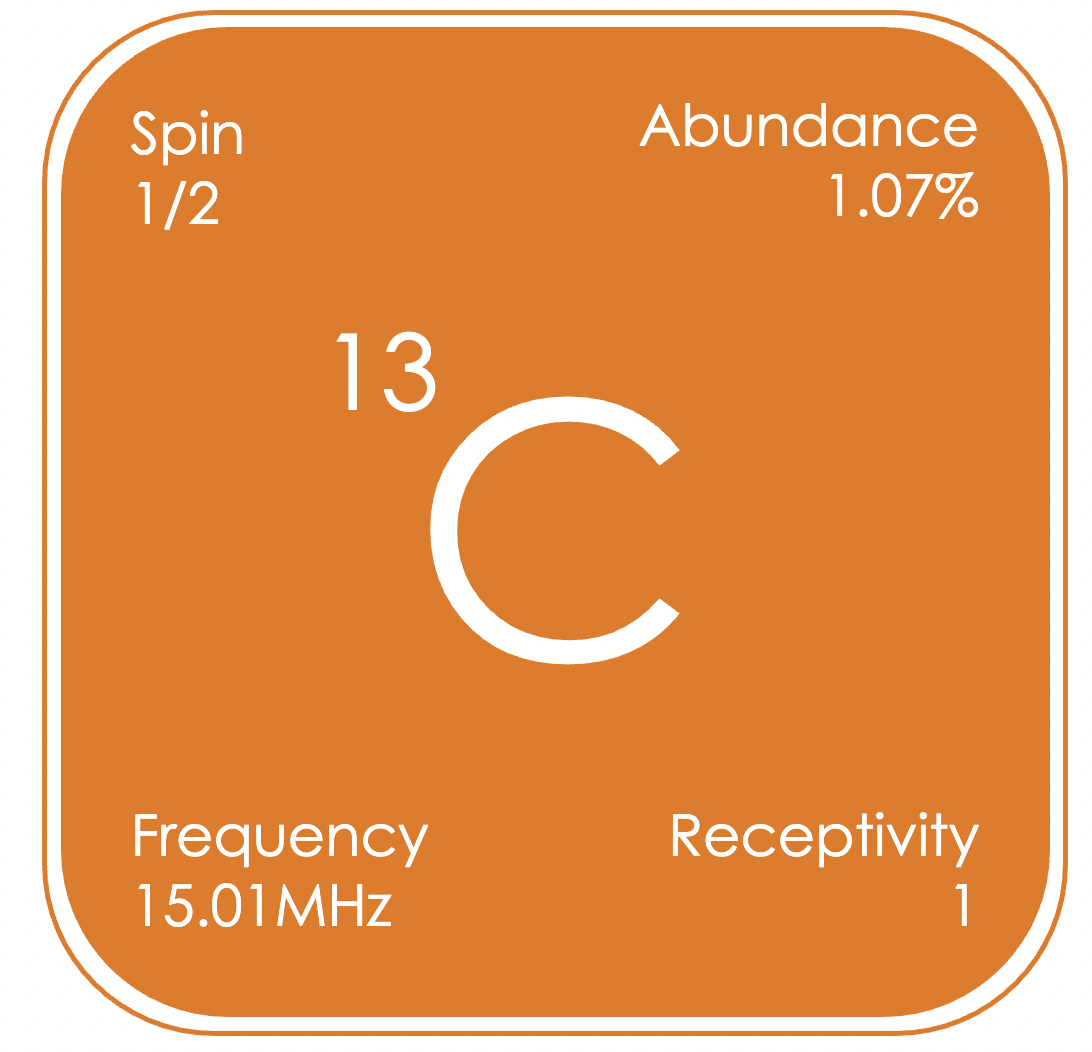
¹³C is likely the second most studied nucleus by NMR spectroscopy, after protons, due to the importance of carbon in organic chemistry and the life sciences. However, it has one of the lowest receptivity values of any NMR active nuclei. Indeed, if not for its ubiquitous nature, ¹³C would likely be an uncommonly studied nucleus by NMR spectroscopy, rather than one of the driving forces in its development.¹³C NMR spectra are usually obtained using a technique known as decoupling, to remove the proton-carbon couplings, greatly simplifying the spectrum and enhancing the signal-to-noise ratio. Most small organic molecules typically produce proton-decoupled ¹³C NMR spectra made up of a series of sharp singlets over the chemical shift range of δC 0 to +200 ppm (relative to (CH₃)₄Si in CDCl₃).
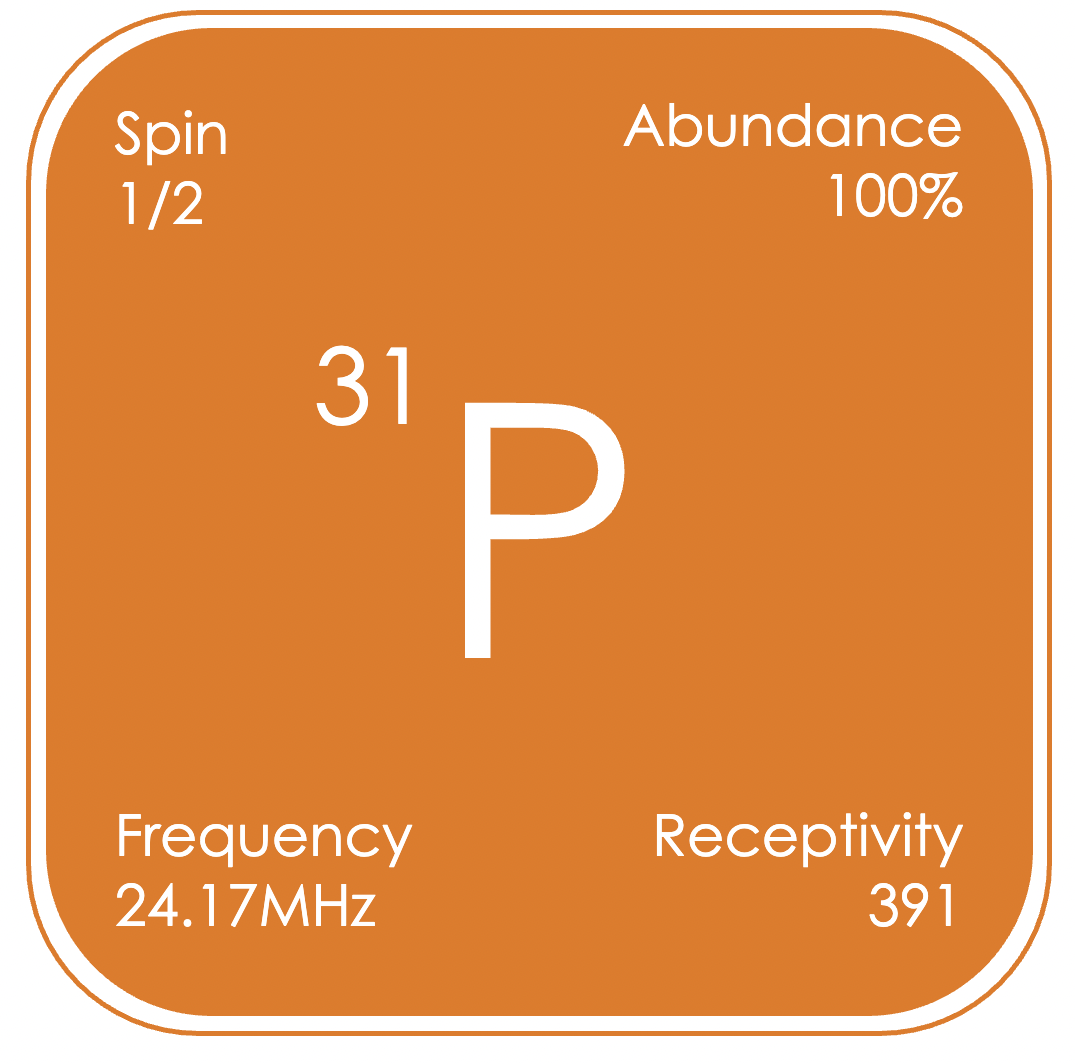
³¹P NMR spectroscopy is commonly used in coordination chemistry, and hence catalysis, where phosphorus containing ligands are often employed. Also, due to the importance of phosphate (PO₄³−) in many biochemical pathways, phosphorous containing compounds are found in pharmaceuticals and dietary supplements which are amenable to study by NMR. Additionally, there are many phosphorus containing anions, for example hexafluorophosphate, which are commonly used in lithium-ion batteries. ³¹P spectra are commonly proton-decoupled, giving generally sharp signals over a wide range of δP −180 to +250 ppm (relative to H₃PO₄ in D₂O).

¹¹B is a quadrupolar nucleus, its spin of 3/2 leads to generally broader signals than spin 1/2 nuclei, with the linewidths strongly dependent on the size of the molecule and symmetry around the boron centre, which are observed over a range of δB −120 to +90 ppm (relative to BF₃·OEt₂ in CDCl₃). Boron containing compounds have a range of applications, including as Lewis acid catalysts and in Suzuki cross-coupling reactions ; hence, they are commonly used as intermediates in the synthesis of fine chemicals and pharmaceuticals.
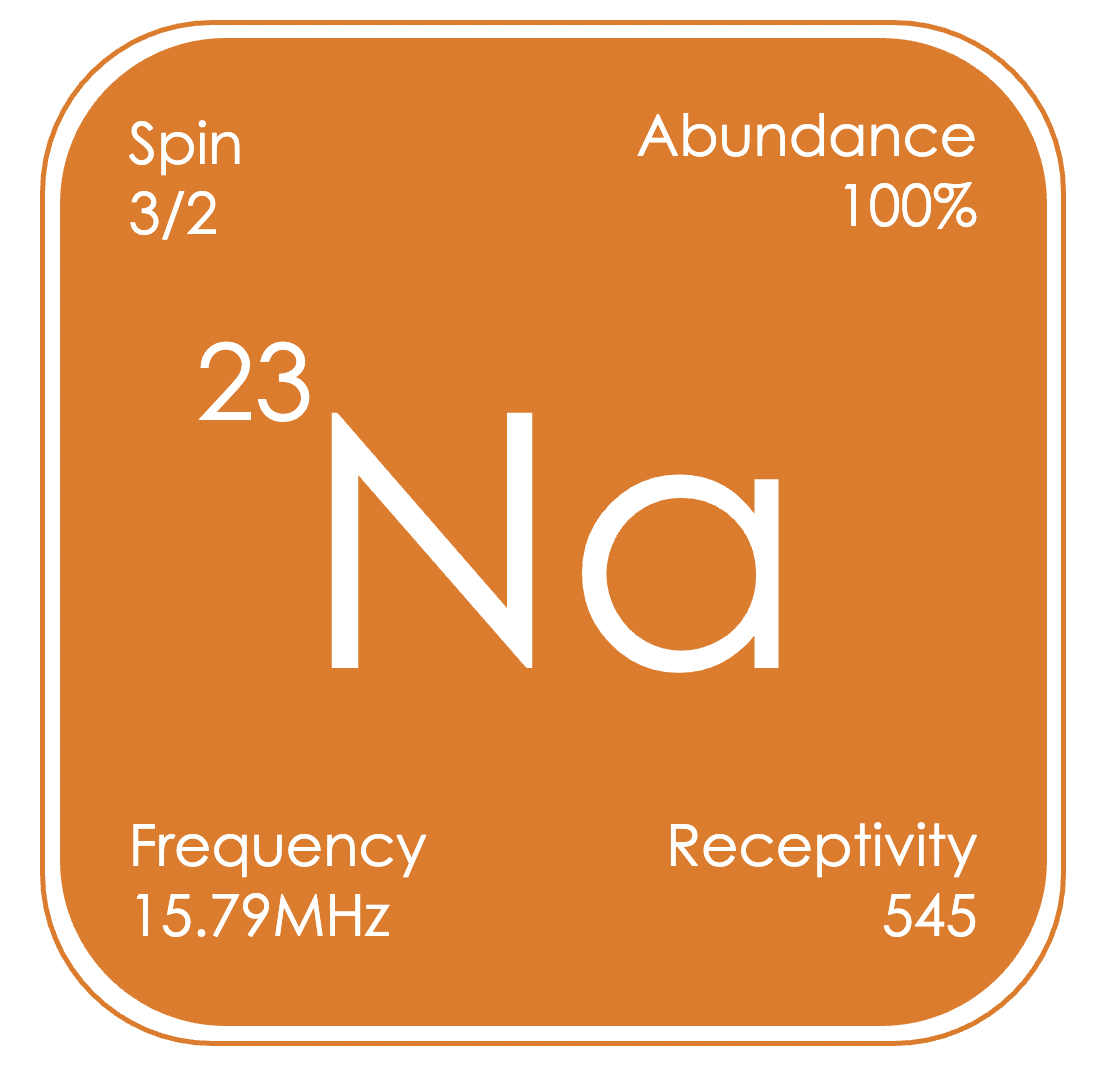
Sodium is the sixth most abundant element on earth and is ubiquitous in living organisms. Sodium containing compounds are valuable reagents in organometallic chemistry. Moreover, sodium is a component of an extremely wide range of substances from food and drink to pharmaceuticals, petroleum additives, and polymers. ²³Na is a quadrupolar nucleus, with a linewidth strongly dependent on the local symmetry and the size of the molecule. ²³Na signals can be observed over the range of δNa +10 to −60 ppm (relative to NaCl in D₂O).
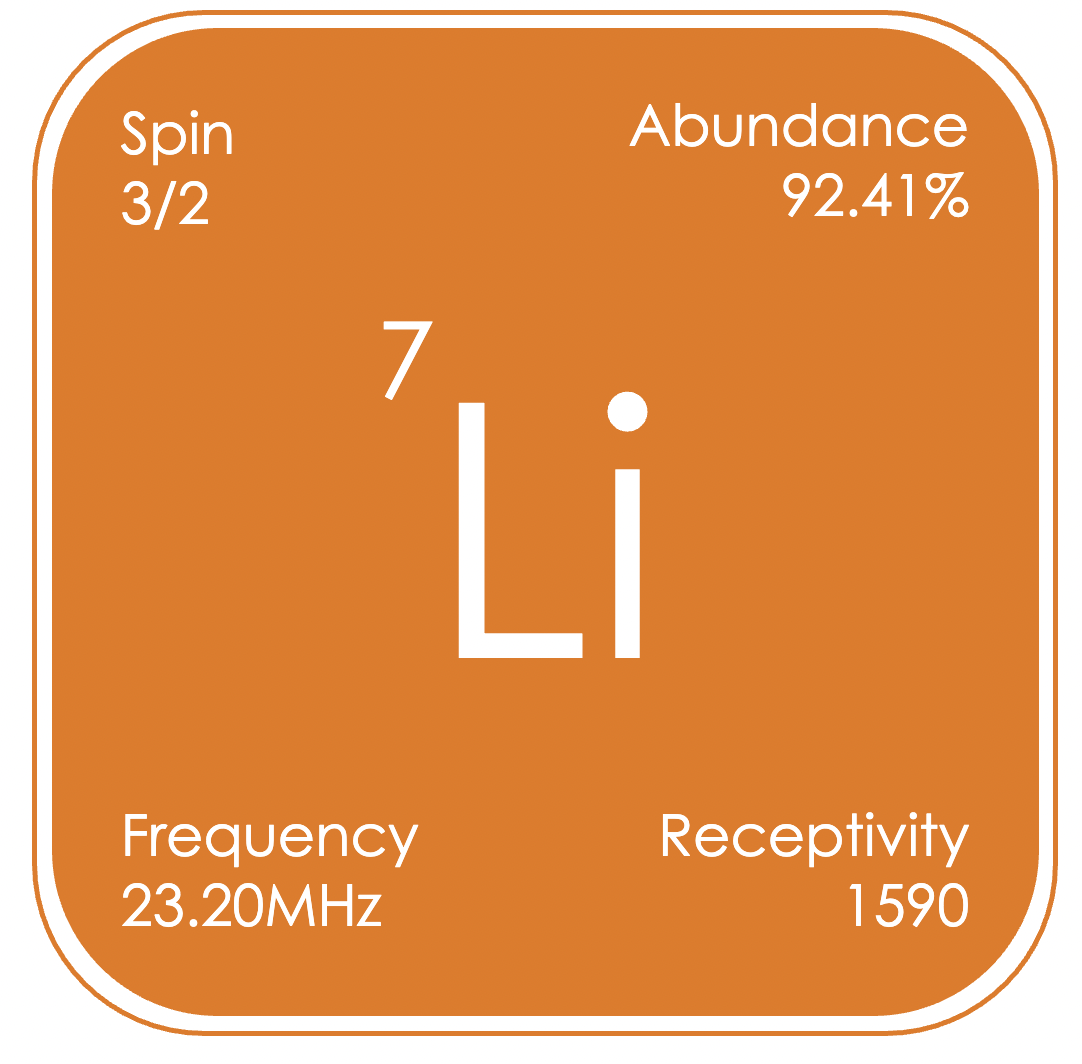
Lithium containing compounds are valuable reagents in organometallic chemistry, although lithium does not have a known biological role, it is used in pharmaceuticals. One of the rapidly growing applications of lithium is in energy storage systems, and solutions containing Li+ cations, such as those used in batteries, are amenable for study by NMR spectroscopy. ⁷Li is a quadrupolar nucleus with linewidths strongly dependent on molecular symmetry and size. ⁷Li signals appear over a range from δLi +15 to −20 ppm (relative to LiCl in D₂O).

²⁹Si NMR spectroscopy is useful for a range of applications, including the analysis of poly-siloxanes (silicones) and their monomer precursors, which have a range of uses including as adhesives, lubricants, and sealants, along with uses in medicine and in the home. ²⁹Si has a low receptivity, and signals appear over a range of δSi −350 to +175 ppm (relative to (CH₃)₄Si). Methods originally developed for sensitivity enhancement in ¹³C NMR are therefore commonly used in ²⁹Si NMR. Such as the use of the DEPT (Distortionless Enhancement by Polarisation Transfer) pulse sequence, where the higher receptivity protons, rather than the ²⁹Si nuclei, are excited.
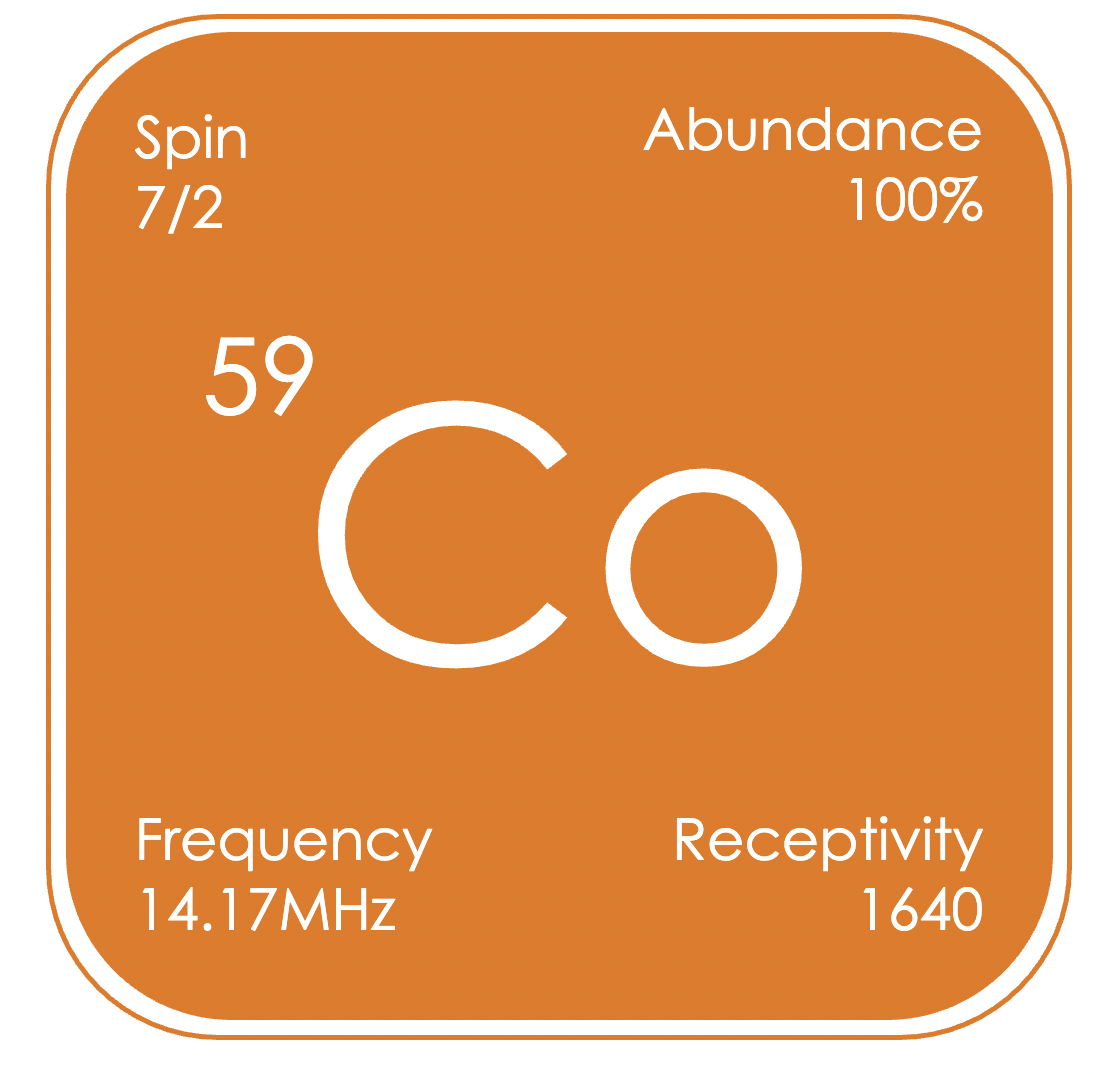
⁵⁹Co played an important role in the development of NMR spectroscopy, as the first nucleus for which the variation in chemical shift for different compounds was observed. Since many cobalt compounds are paramagnetic, ⁵⁹Co NMR spectroscopy is generally limited to low-spin cobalt(I) and cobalt(III) complexes, which are observed over the largest chemical shift range of any nucleus, δCo −4,000 to +14,000 ppm (relative to K₃[Co(CN)₆] in D₂O).
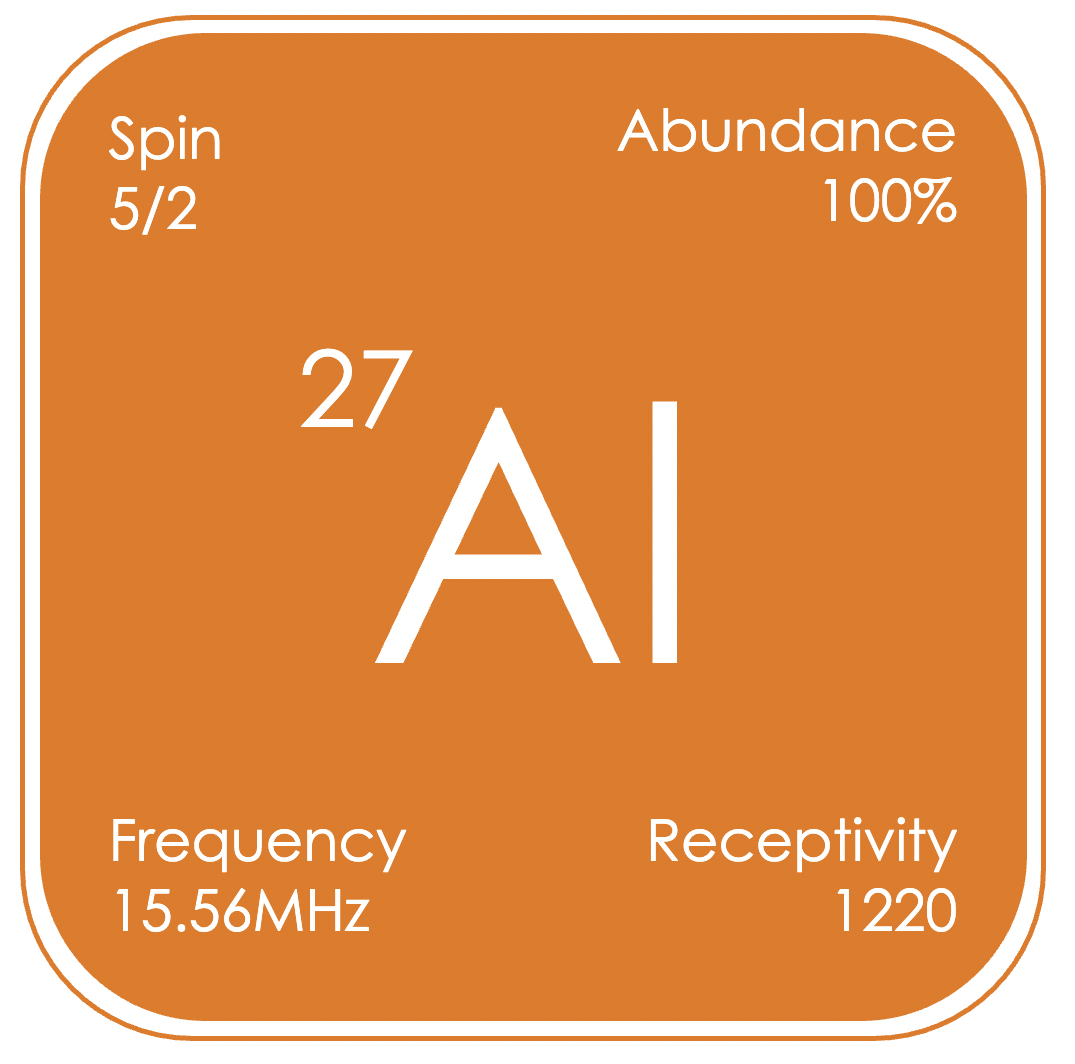
²⁷Al is a high receptivity nucleus, which gives generally broad signals over the range of δAl −200 to +200 ppm (relative to Al(NO₃)₃ in D2O). Aluminium containing compounds are common reagents and intermediates in the synthesis of pharmaceuticals and fine chemicals; and there are uses of aluminium for energy storage, with Al-ion batteries having the potential to replace Li-ion batteries, due to their higher theoretical energy densities and the higher abundance of aluminium in the Earth’s crust.
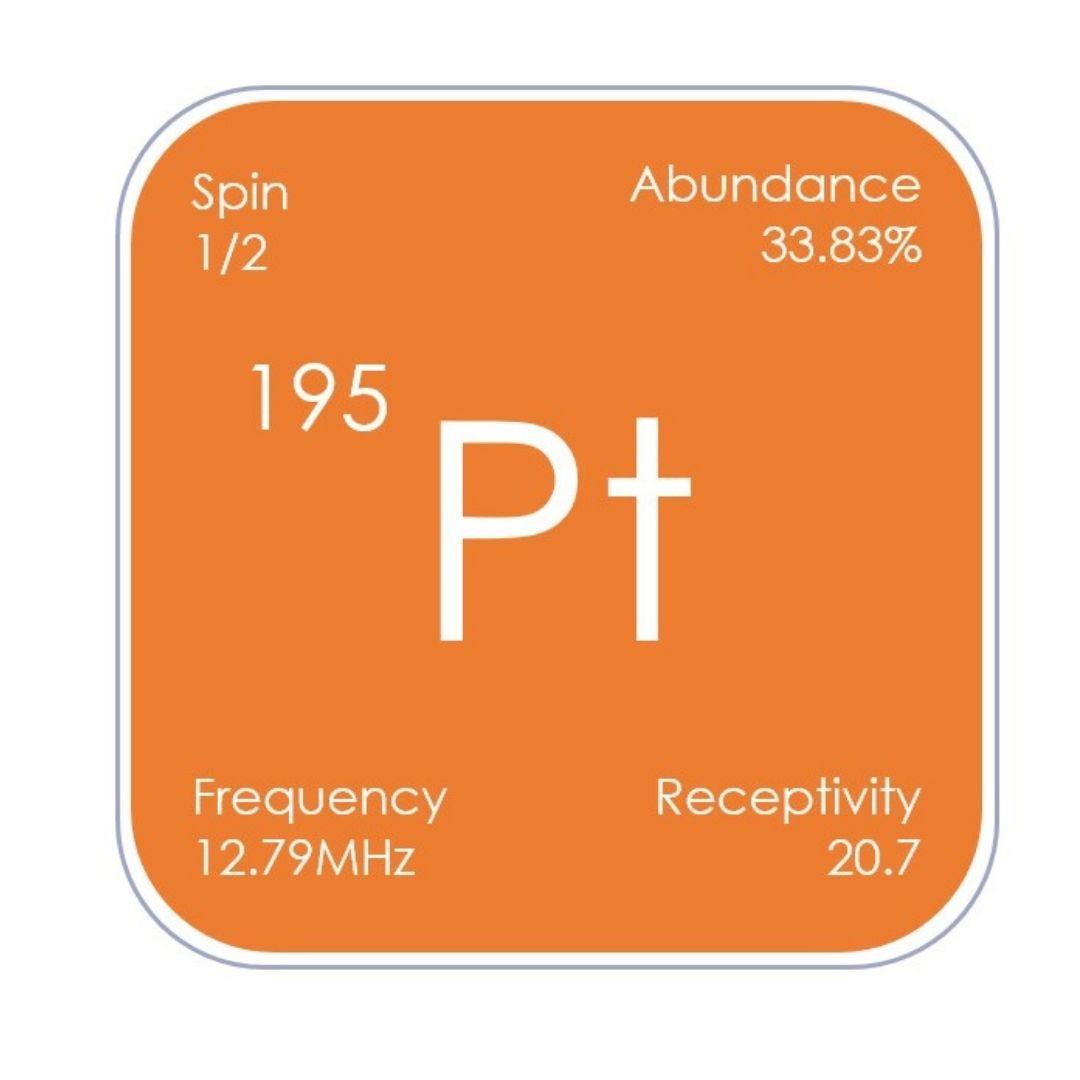
Platinum containing compounds have many applications, including as pharmaceuticals and catalysts, and 195Pt NMR spectroscopy is widely used for studies of these systems. 195Pt signals are observed over a wide range of 15,000 ppm (relative to Na2[PtCl6] in D2O at δPt
0 ppm), with the chemical shift strongly dependent on the oxidation state and coordination environment of the platinum centres.
For example, spectra of the half-sandwich platinum(IV) complex, [Pt(η5-C5H4CH3)(CH3)3], show a distinct signal in the 195Pt{1H} NMR spectrum at δPt −5240 ppm, with coupling to the nine-equivalent protons of the three methyl ligands is clearly resolved in a proton-coupled 195Pt spectrum. While in the corresponding proton NMR spectrum, signals with distinct platinum-satellites from the 34% abundant 195Pt are observed.
