Products
Applications
Learning
The The X-Pulse benchtop variable temperature NMR spectrometer is capable of true variable temperature operation, with the sample temperature varied through the use of a temperature controlled external gas flow. This allows sample temperature to be controlled from +0°C to +65°C on X-Pulse, and from +0°C to +60°C on X-Pulse 90. Temperatures outside this range are achievable dependent on the specific installation. All this is possible while maintaining a magnet temperature stability within 0.001°C to ensure optimum spectral quality.
The capability to obtain NMR spectra at various temperatures is valuable for a wide range of applications, for example: the direct measurement of temperature dependent physical properties by NMR; allowing for the analysis of systems which are only stable in a specific temperature range; the investigation of the thermodynamics and kinetics of chemical processes in solution; and being able to consistently obtain measurements using a range of techniques under the same conditions. Some examples of these are detailed below.
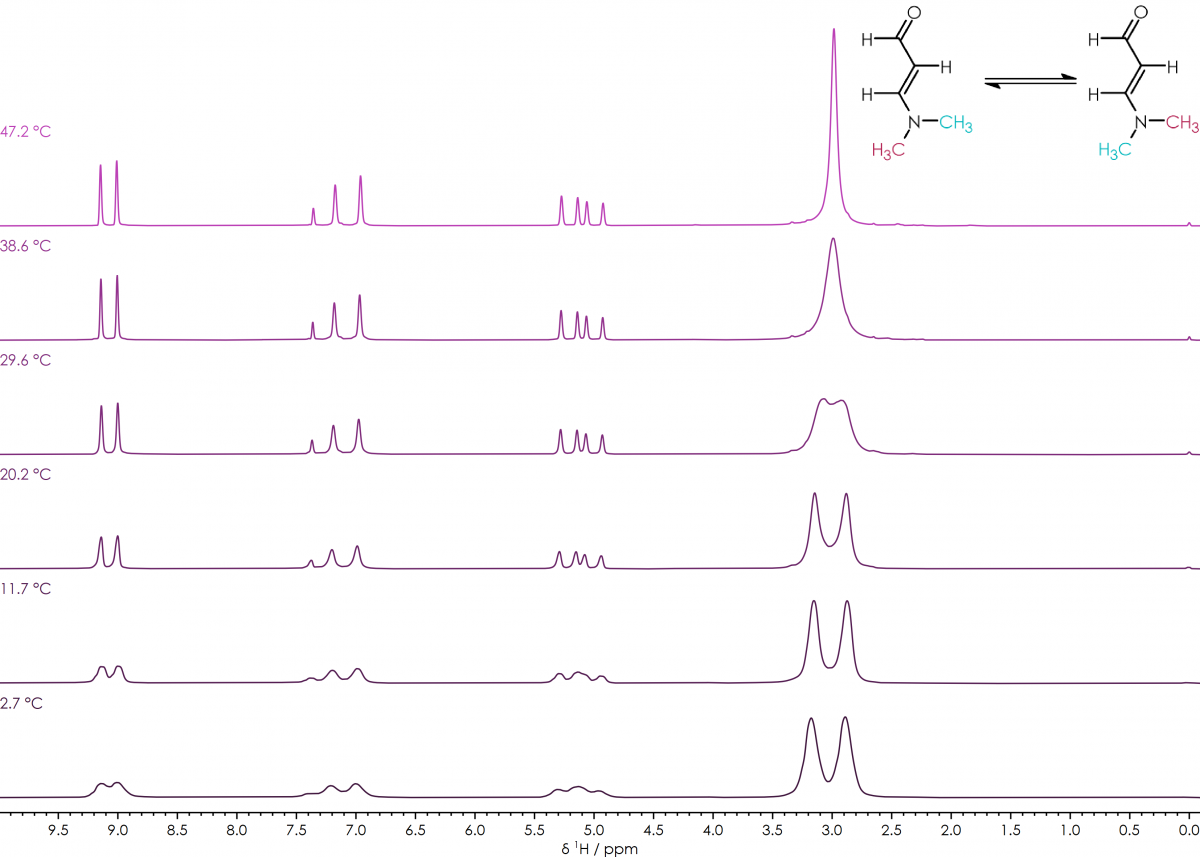
¹H NMR spectra of 3-Dimethylaminoacrolein in CDCl₃, over a temperature range of +2 to +48°C
There are numerous chemical processes which occur in solution, where variable temperature NMR spectroscopy can be used to monitor these processes and extract thermodynamic and kinetic parameters.
One example is the cis-trans isomerisation of the amino-methyl groups in 3-dimethylaminoacrolein, where the timescale during which the isomerisation takes place, is comparable to the time scale of the NMR experiment allowing it to be easily observed. At low temperatures rotation about the carbon-nitrogen bond is slow and the two methyl groups give separate peaks in the ¹H NMR spectrum; while at higher temperatures faster rotation leads to the two methyl groups appearing as equivalent by NMR giving a single peak. From the coalescence of the two peaks as the temperature increases, it’s possible to calculate the thermodynamic barrier for rotation of the carbon-nitrogen bond.
Would you like to learn more about measuring kinetics with benchtop NMR?
Ask us a questionThe knowledge of the diffusion constant of a species in solution is valuable for a number of applications, particularly for the analysis of the constituents of liquid electrolytes in batteries and other electrochemical devices. Diffusion constants can be directly measured by NMR spectroscopy through use of the Pulsed field Gradient Spin-Echo (PGSE) pulse sequence (see Application Note 17 for more details), and display significant temperature dependence.
Therefore, it is essential for a complete understanding of a system, for the diffusion constant to be directly measured across the entire temperature range where a device would be expected to operate. Using the X-Pulse broadband benchtop NMR spectrometer in variable temperature mode allows for diffusion constants to be directly measured for all the nuclei commonly found in battery electrolytes (¹H, ⁷Li, ¹¹B, ¹⁹F, ²³Na & ³¹P), across the entire standard operating range of modern Lithium-ion batteries. For example, series of PGSE spectra obtained of a solution of Li+ over a 55°C temperature range; show how as the temperature increases, the attenuation of the signals becomes more apparent at lower gradient strengths (indicative of increased diffusion constants as the temperature increases).
Would you like to learn more about understanding temperature dependence of self diffusion?
Speak to the team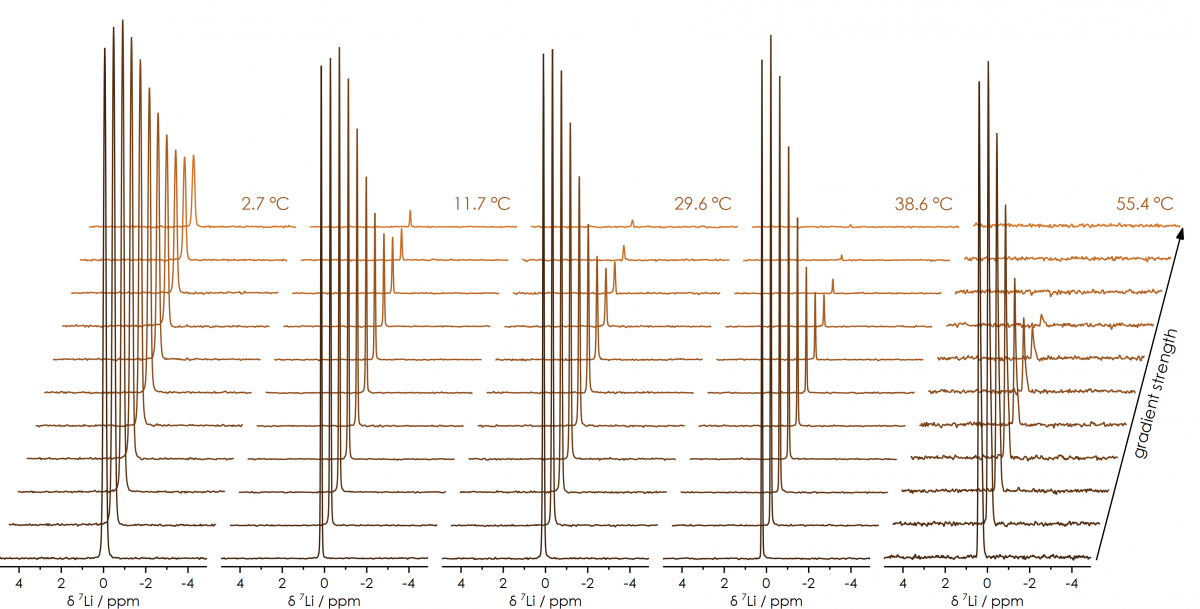
series of ⁷Li PGSE spectra of Li+, over a temperature range of +2 to +56°C
In an NMR spectrum, the majority of signals in simple diamagnetic systems are relatively insensitive to variations in temperature. One notable exception is the chemical shift of the hydroxide (and hence water) signal, which varies significantly with temperature. By choosing a suitable pure compound which combines a hydroxide and alkene signal, it is possible to calculate the temperature of the sample from the chemical shift difference (Δδʜ) between the two signals. Ethylene glycol and methanol are commonly used in this role, and the equations to calculate the sample temperature from the spectra are well established (J. Magn. Reson., 1982, 46, 319-321).
Ethylene Glycol (HOCH₂CH₂OH): T(°C) = 193.35 – 102.00 × Δδʜ
suitable range: 0 - +140 °C
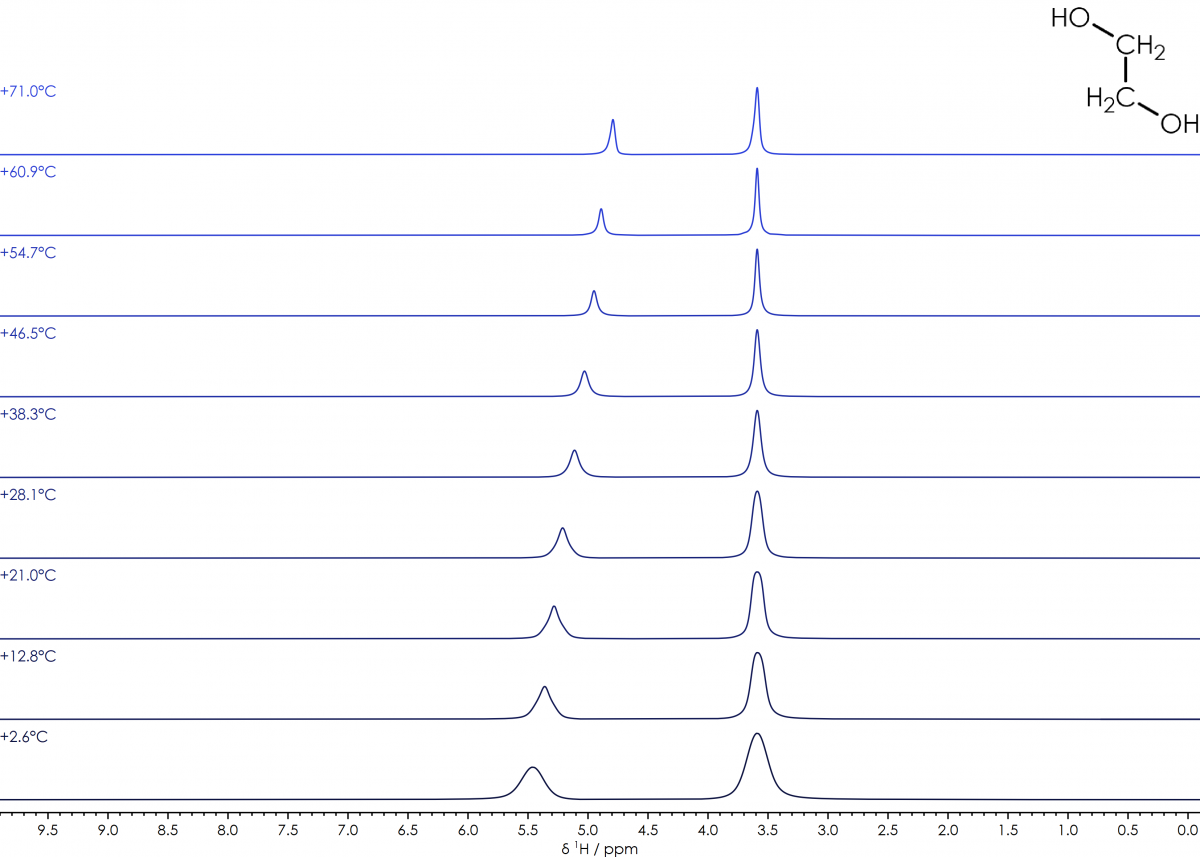
¹H NMR spectra of 100% Ethylene Glycol over a temperature range of +2 to +71°C
Methanol (CH₃OH): T(°C) = 135.85 – 36.54 × Δδʜ – 21.85 × Δδʜ 2
suitable range: −95 - +57 °C
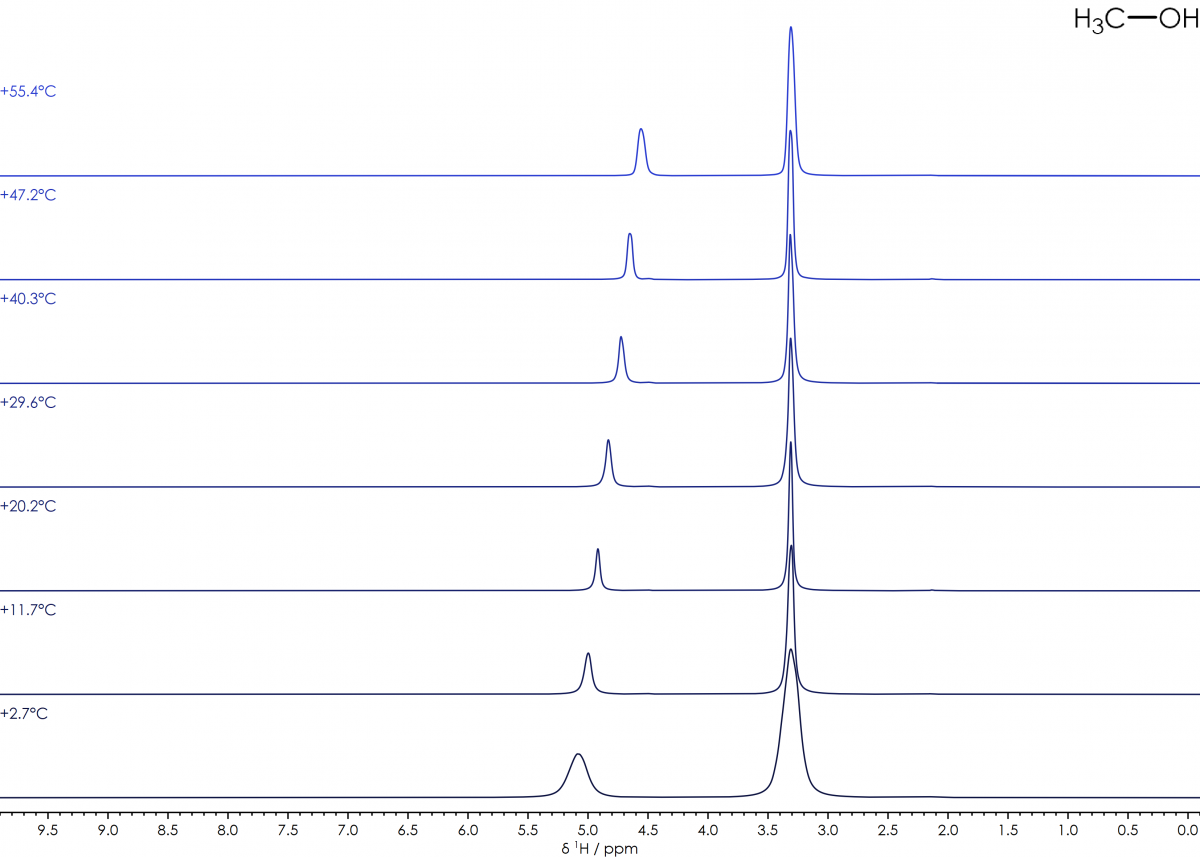
¹H NMR spectra of 100% Methanol over a temperature range of +2 to +55°C
Would you like to learn more about measuring temperature sensitive samples with benchtop NMR?
Ask us a question