Products
Applications
Learning
Low-cost and high-accuracy is essential for effective polymer characterization, providing an opportunity for low-field nuclear magnetic resonance (NMR) spectroscopy to replace costlier and destructive methods.
In this recent webinar, Dr. Bryan S. Beckingham, Assistant Professor at Auburn University, and Marcel Lachenmann of Oxford Instruments describe how NMR spectroscopy characterizes polymer composition, microstructure, and physical properties and monitors synthesis reactions.
Read on for highlights from the live Q&A session or watch the full webinar on-demand.

Marcel Lachenmann, Oxford Instruments

Dr. Bryan S. Beckingham, Auburn University

Q: What is the variable temperature (VT) range on the X-Pulse? Is it possible to analyze samples that are above the VT range?
ML: The VT range on the X-Pulse is 20 to 60 degrees Celsius which is useful for a lot of applications such as polymers, and other types of chemicals as well. Not all of them can be dissolved at that low of a temperature. It is possible to go somewhat higher than the variable temperature limits. We have data at 72 degrees, but we haven't tested all of the limits of where we can go at the high end. 200 degrees would be difficult, but we're more than happy to discuss different temperature ranges.
Q: Can the integrations and calculations be performed automatically to eliminate the need for manual calculation by users?
ML: Yes, the integration and calculations can be performed automatically. The Mestrelab Mnova processing software supplied with the instrument can be scripted so you can use scripts for completely automatic processing.
Q: What are the typical average molecular weights of the polymers you analyze and what is the highest molecular weight polymer that you think would be possible to analyze?
BB: Most of the materials that we make can be from 5,000 grams per mole at the low-end to 150,000 grams per mole at the high-end. For the low field, we haven't tested beyond that. We have run cross-linking network polymerizations inside NMR tubes in the Pulsar where we aren't tracking the polymer necessarily, but we're tracking the monomers, and those work all the way up until it's past its gel point. I’m not sure that there's a good practical limit other than what you can get in an NMR tube.
Q: Are you aware of low-field NMR applications for vinyl copolymers, blends, and alloys, and is the sensitivity good enough?
ML: There are certainly applications for vinyl co-polymers, and I'd like to talk to you about the blends and alloys. We're interested in learning more about the kinds of applications in the polymer industry that people are interested in. Is the sensitivity good enough? That is an excellent question and it really does depend on the sample and the application. For example, although this isn’t a vinyl copolymer, you can do poly(styrene-co-butadiene) polymers and polymer composition, but it will depend on the type of levels that you're looking at and the type of sample that you're using. This is something that we would love to discuss with people.
BB: I will add more to the vinyl co-polymer systems. I'm assuming that we're talking about polypropylene. We've done some work with an industrial partner looking at some polypropylene in the low field instrument. Propylene is hard to do with NMR because of solubility issues so you run into the same problems that you run into at high field. The sensitivity for those systems is in the aliphatic region which is messier for polymers in general.
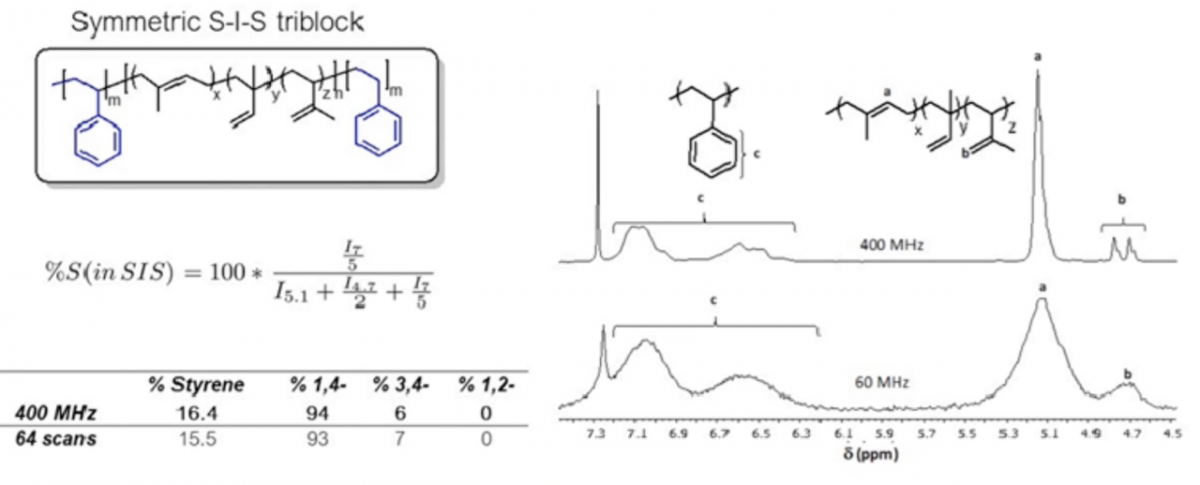
Q: In the NMR of polystyrene, why are there no higher field absorptions due to the aliphatic nature of the C and H?
BB: There are aliphatic peaks for the polystyrene and they're down about 1.9 ppm or so. They're in the one to two ppm range for those backbone CH2 groups. We tend to not use them very much in the analysis because when we're doing copolymers and blends. It's easier to use the vinyl groups for integrations. They can be used, but as I was saying to the last question, the aliphatic region is much more complex for polymers, especially for hydrocarbon polymers like these. However, they're there; I have just cropped them out of a lot of the spectra I show here.

ML: That's an excellent example of the way benchtop NMR can be slightly different from the way that a lot of people think of NMR - that you're concentrating on what you can use to get the information most easily. You don't have to look at the whole spectrum, what you do need to look at is the part of the spectrum that's easy to get at, and that gives you the answer you need.
Q: Will peak deconvolution help in peak resolution for this benchtop NMR?
ML: We have done a lot of peak deconvolution and yes, it does help you in analyzing the data for certain samples. It will depend on the actual sample, but reference deconvolution worked well for us. We've also used the Mnova software which has global spectrum deconvolution. As a spectroscopist rather than a mathematician, I tend to trust the data more than I do the mathematical algorithms. I can speak from experience that it is a useful technique, and it does work well if you're careful in applying deconvolution.
Q: Did you acquire all the polymer data as neat or did you use any solvent?
BB: All the samples I showed here were in deuterated chloroform, which is our standard go-to solvent for most polymers like these.
ML: Since polymers tend to be solid, you do need to dissolve them in something for the X-Pulse because it only does solution state or liquid samples. As Brian mentioned, you don't necessarily need to use deuterated solvents for polymers; you can use protonated solvents if they’re not right on top of a peak that you need. For relaxometers such as MQC+, those samples are not dissolved in anything. The polymers are measured neat so you can dump a solid into the tube and get good relaxometry data out of it.
BB: The only reason we use deuterated chloroform here was because we were running the same samples in the high field which required the deuterated solvent. We do routinely do these analyses just in normal chloroform and we get just as good data.
Q: Which temperature do you dissolve your polymers? Are high-temperature measurements possible on a benchtop instrument?
BB: Ours were all dissolved at room temperature. The Pulsar doesn't have the temperature control that the new X-Pulse does and so all our measurements were done at the Pulsar internal temperature, which is about 37 degrees C.
ML: You can do that without having temperature control – you can heat up a sample and put it into the Pulsar. It's going to lose temperature as you're measuring so it works best for a quick measurement. If you're going to need several scans, then that adds complications. It is possible to do higher temperatures.
Q: Is the benchtop instrument usable for real-time continuous measurements, and how long does a single measurement take?
ML: This is going to depend on what your application is, but yes, you can do a continuous measurement. How long an average measurement takes is again dependent on how much polymer you've got in solution, what's the concentration and what is it exactly that you're looking for in your application. It can go from a single scan to 16 or 32 or more scans, depending on the level of signal to noise that you need to achieve.
BB: Most of the standard measurements we run are about 30 minutes.
ML: The funny thing about flow is you can cut down repetition times to some extent because you don't have to worry about T1 the same way. For polymers, you generally don't have to worry about T1 too much because they relax very quickly. As a result, there are ways of cutting down the measurement times. Some of the polymer applications that I've done in the past have typically been on the order of 10 minutes for the acquisition time. However, that was because we were looking at relatively low signal levels. If you were interested in a composition of something where the components that you're looking at have very high signal levels, you could get away with a few seconds for your measurement.
BB: We do reaction kinetics where we do a lot less scans. There we only care about the monomer and so if you're just doing the small molecules for something like real-time measurements of reactions, you can get that down to under a minute and have plenty of signal.
X-Pulse: high resolution, broadband benchtop NMR spectrometer
X-Pulse uses a 60MHz permanent magnet with high homogeneity and temperature stability and no liquid cryogens, making it easy to position in almost any academic or industrial chemistry laboratory.
Ask a Question