Products
Applications
Learning
4th January 2024 | Author: James Sagar
NMR is a key analytical technique in many industries, whether academic research, pharmaceuticals, polymers or batteries. Mostly when people think NMR in these spaces, they think of solution state NMR and quite often are thinking of it for the smaller organic molecules or at least mixtures of those. When we think about benchtop NMR this become even more focused on small organic molecules, you would surely not think about putting actual solids into the instrument and get anything like the useful information that you could get from high field solid state NMR with magic angle spinning (MAS)?
Obviously, you are right.
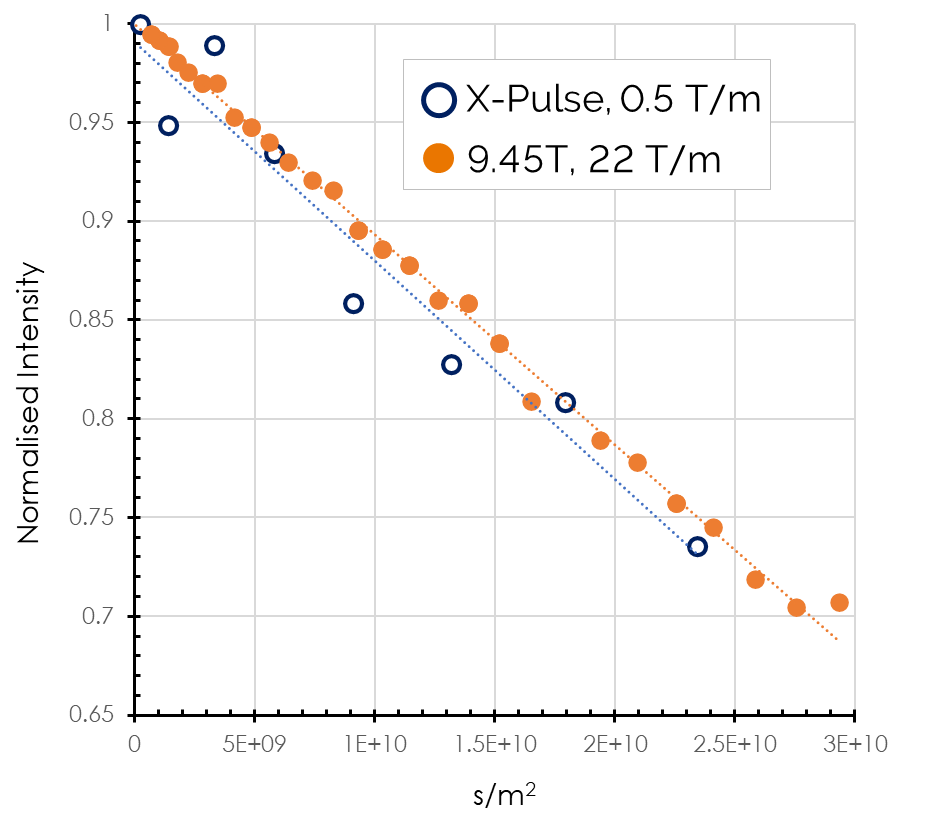
However, not all solid-state analysis requires detailed/precise (high resolution) MAS spectra. There are applications in analysis of solids where benchtop NMR instruments and their small form factor give a significant incentive to do so. What we have to do is find something that acts a little bit more “liquid-like” even if it is generally considered a solid. One group of materials where this can be found to be true is in solid state electrolytes for future generations of rechargeable batteries. The holy grail of an all-solid state battery with long lifetimes if an enticing prospect and it isn’t surprising that that they are currently a field of intense research interest. For the purposes of benchtop NMR the one thing that we can tap into is the fact the lithium ions have to be mobile otherwise it would be a poor electrolyte, this mobility means they are slightly more “liquid-like” and therefore we can still generate useful information. One key piece of information is the mobility of the lithium which in turn determines the conductivity of that electrolyte. To measure this, we can use an NMR spectrometer fitted with pulsed-field gradients and a pulsed-filed gradient stimulated echo sequence (PFGSTE) to directly measure the diffusion coefficient of the lithium ions. There are a wide range of materials being considered for this application from polymers to ceramics and as such have a wide range of expected lithium diffusion coefficients and therefore conductivities. Polymer salt mixtures can have high diffusion coefficients in the range of 10−-7 (cm2.s−-1) whereas pure polymer electrolytes such as LiPON (lithium phosphorus oxynitride, LixPOyNz) are closer to the 10−-11 range, the perovskite or ceramic or garnet electrolytes such as LLZTO (lithium lanthanum zirconium titanium oxide, Li6.4La3Zr1.4Ta0.6O12) meanwhile can be as low as the 10-18 range. One family of materials that is potentially closer to commercialisation than others are the Argyrodites with large companies such as BASF bringing these to market; these compounds have a nominal composition of Li6PS5Cl however there are small variation on that.
We have recently shown it is possible to measure lithium diffusion in these compounds using benchtop NMR, with very good agreement with high-field solid state NMR. Using both techniques we were able to measure a diffusion coefficient of (5.9±0.7)×10−-12 cm2.s−-1. This is almost at the limit of what is possible with current benchtop NMR spectrometers, however it asks the question do we need a NMR spectrum or is the diffusion data enough? If the answer is we just need the diffusion data then we can begin to consider time-domain NMR techniques where it is possible to achieve significantly higher pulsed field gradients while still having the instrument on the bench in a conventional lab.
Want to learn more about our benchtop NMR solutions? Click through the link.
We would like to thank Dr Gregory Rees and colleagues from the Sir Peter Bruce Group at the Department of Materials, University of Oxford whose contributions and collaboration were integral to the successful development of this application.
Dr James Sagar,
Materials Analysis Group Business Manager, Oxford Instruments
James Sagar has been a Strategic Product and Applications Manager in benchtop NMR at Oxford Instruments since January 2019. He joined the company in 2015 as Product Manager for energy dispersive X-ray analysis, looking after the world’s first EDS detector for electron microscopes capable of detecting Li X-rays. Before this, James carried out post-doctoral research at University College London.