Products
Applications
Learning
In this interview, we talk to Dr. James Sagar, our Strategic Product and Applications Manager, about how benchtop NMR can be used to optimize battery electrolyte performance and quality.
Can you give us an introduction to the services that Oxford Instruments provides to the battery industry?
We have a strong focus on batteries at Oxford Instruments. The batteries we supply to the battery industry address a range of analytical solutions, from quality control to component manufacturing and from raw materials processing to initial exploration. Oxford Instruments is the leading supplier of electron microscopy-based analysis solutions. Our solutions are used by our customers for a range of applications, including high-end research into the development of novel materials for solid-state batteries and assessing the quality of battery raw powders and materials for battery electrodes.
We use nanoscale surface characterization with our atomic force microscopes to allow our customers to understand how the performance of battery materials can impact electrochemical processes. However, the focus of this interview is our Nuclear Magnetic Resonance (NMR) spectrometers. These are used in product development for current-carrying liquid electrolytes and for addressing quality control.
What is the difference between a broadband benchtop NMR system and a traditional NMR setup?
The thought of NMR often brings to mind a large cylinder in a specialized room, requiring expertly trained operators and the use of liquid cryogens. We have shrunk that technology down into an instrument that can sit on a bench in a typical lab with benchtop NMR, although it has a reduced magnetic field strength. It is now possible for us to efficiently perform most of the same experiments that once required a traditional high field NMR system thanks to these lower-cost, compact systems.
In the development of next-generation batteries, broadband benchtop NMR is an incredibly powerful technique. Just one instrument is needed to analyze the wide range of elements of interest in battery electrolytes thanks to broadband.
This is a new technology and can be utilized in the predication of electrolyte performance within end product battery systems and for the development of new formulations.
What instrument performance and other considerations impact the choice of benchtop NMR system?
One of our benchtop NMR systems should be able to operate without liquid cryogens in any standard lab at room temperature. A highly trained, experienced, or dedicated operator should not be required.This enables users to step in and acquire data as and when they need to. Lastly, the machine’s dimensions must allow it to be placed in an inert glove box to allow characterization of air-sensitive battery materials, be transported around on a trolley, or fit on a bench.
All peaks of interest in your spectra should be discriminated between by the instrument’s spectral resolution. Sufficient sensitivity is needed for the detection of key species in your materials. To repeatedly make accurate measurements, stability is also needed. A true ‘broadband’ system is required to be able to analyze all the relevant chemical nuclei in a formulation for electrolyte characterization. This is advantageous for ensuring the best possible understanding of the materials.
In some advanced experiments, it is important to have the potential to exploit pulsed-field gradients. The use of these gradients enables quantification of transference (how the current-carrying ions migrate as a function of their charge), ionic conductivity, and diffusion coefficients. Finally, it can also be important to be able to use variable temperature control for the investigation of electrolytes over common battery operating ranges.
How does the X-Pulse benchtop NMR spectrometer help meet these needs?
Our latest benchtop NMR spectrometer is the X-Pulse. It can resolve complex proton spectra at 60MHz field strength thanks to its better than 0.35 Hz spectral resolution at the half-height of a peak.
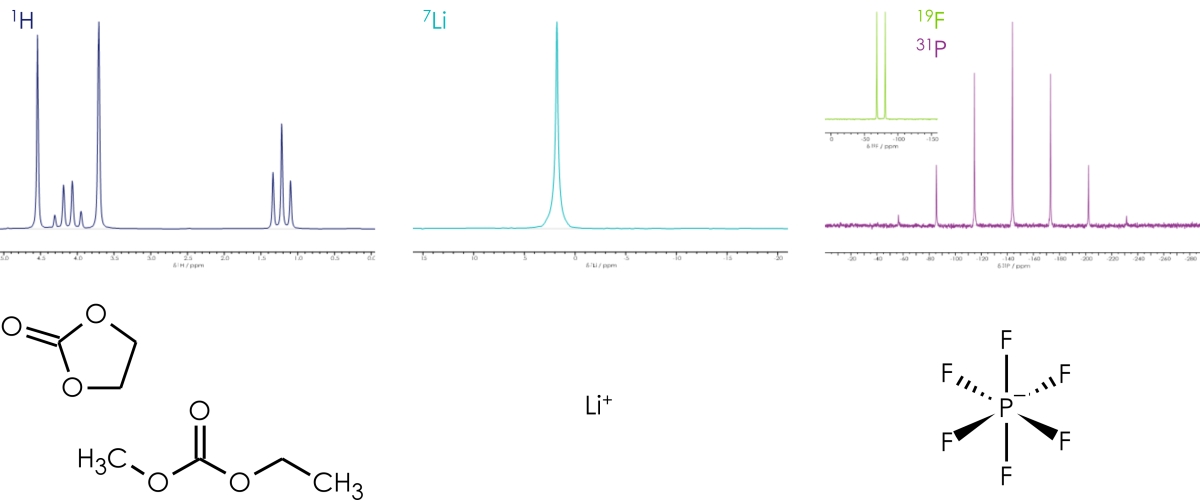
The X-Pulse is unique in that it is the only benchtop NMR instrument with built-in broadband multinuclear capability. This capability allows users to collect spectra from the wide range of nuclei present in electrolytes, including carbon, hydrogen, sodium, boron, phosphorus, fluorine, and lithium. The temperature of the sample can be kept in the typical operating conditions for most batteries, which is between 20°C to 60°C.
The standard pulsed-field gradients can understand key physical properties by measuring the self-diffusion of cation and anion species. The X-Pulse can be potentially integrated into a glovebox, transported on a trolley, or installed in any lab because of its physical dimensions.The precise data repeatability required, particularly in quality control, is enabled through the high stability resulting from the magnet.
How is benchtop NMR employed in the analysis of battery electrolytes?
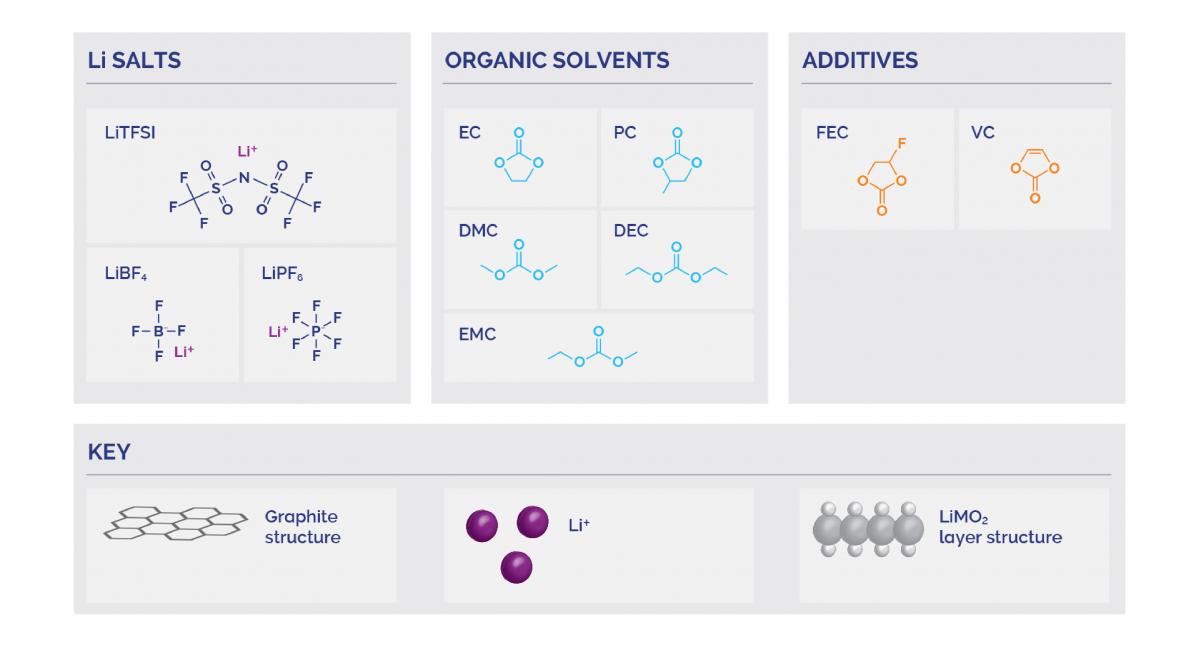
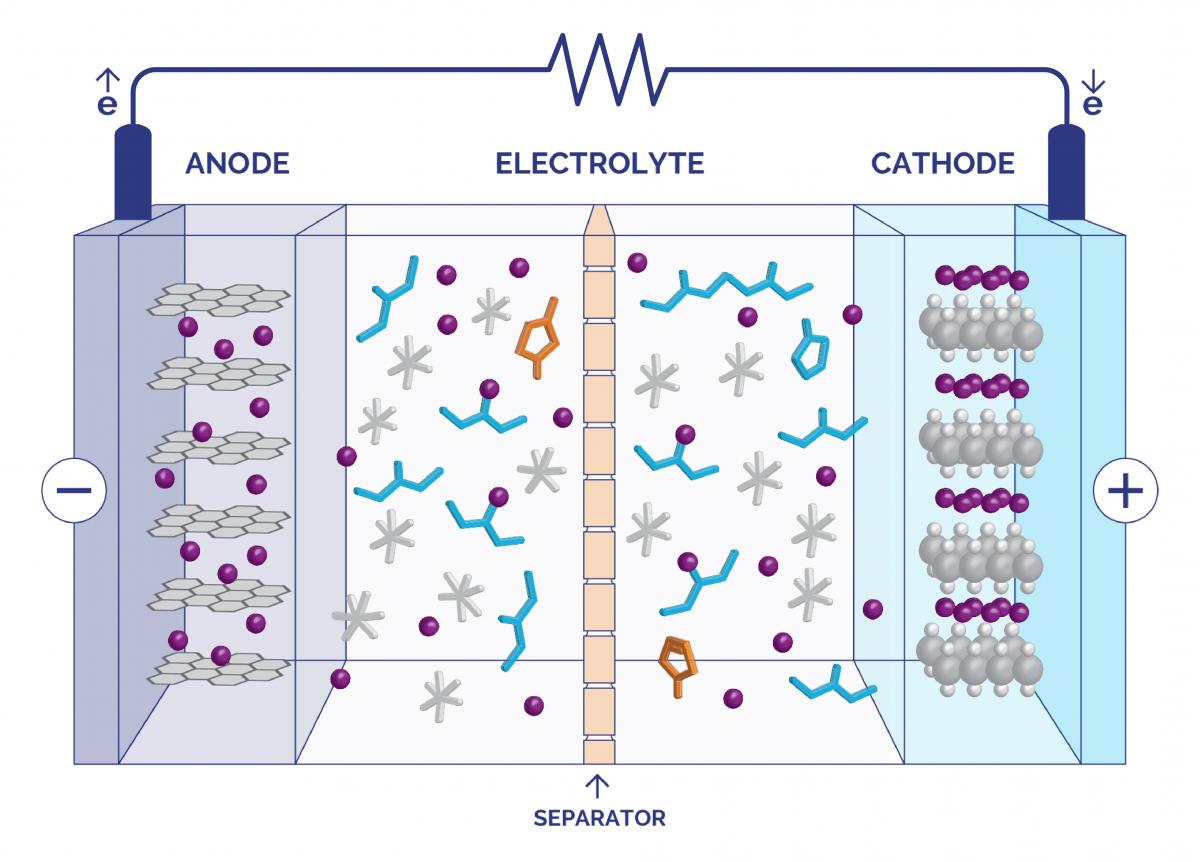
Most batteries will contain a cathode and an anode with electrolytes and an embedded porous separator between them. The electrolyte inside a battery will contain a lithium salt; the most common lithium salts are lithium TFSI ((bis(trifluromethanesulfonyl)imide)), lithium tetrafluoroborate, and lithium hexafluorophosphate.
The salt gets dissolved in an organic solvent – some examples of these include dimethyl carbonate and ethylene carbonate. These can sometimes include additives used to improve the performance of that electrolyte.
The purpose of these additives is to improve the formation of a solid electrolyte interface layer at the electrodes or to enhance stability to prevent decomposition. The passivation layer this creates can cause battery capacity loss but protects the electrodes while still allowing current transfer.
When considering what a good battery looks like, we often break it down into five key areas:
We can measure the salt concentration in electrolytes to better understand energy density and to develop higher power density formulations. We can quantify the transference numbers of those electrolytes and ionic conductivity by determining the diffusion coefficients of the various species in the electrolytes. The cell’s energy density and power, as well as battery lifetime, are impacted by the above parameters as well as the temperature- and time-dependent behavior. Verifying raw materials purity allows us to benchmark electrolytes, allowing us to get rapid feedback on the effect of new formulations for the development of more stable electrolytes and novel additives. To specifically address lifespan, we can monitor electrolyte breakdown reactions to better understand their processes.

Trace levels of water can be used in the acceleration of the formation of unwanted hydrofluoric acid (HF) based breakdown products, for example. Using this process knowledge, we can feed back new information into the development of additives specifically designed for breakdown inhibition. Rapid NMR analysis of novel electrolyte formulations can reduce overheads and accelerate development making batteries more cost-effective. Additionally, the number of defective batteries is significantly reduced, and battery life is extended because of rapid testing that provides a quantification of the quality of a final electrolyte product.
Can you give us a practical example of the use of benchtop NMR in quality control?
A customer wanted to find out if benchtop NMR could accurately determine their solvent composition to help to improve their quality control processes.They specifically wanted to know if it is possible to distinguish between good quality materials and poor quality materials. If it is possible, the next question is to ask what causes failure in poor-quality material? The customer’s samples, in this example, could be described as a combination of ethyl methyl carbonate and ethylene carbonate, and lithium hexafluorophosphate. Two examples, believed to have the same chemistry, were supplied to us.
These two colorless liquid electrolytes had a different performance when placed in a cell, however. We first took a quick hydrogen spectrum and approach most NMR spectroscopists would take with this case. We identified a small peak that we associated with vinylene carbonate (a typical stabilizing agent), as well as both the ethyl methyl carbonate and ethylene carbonate.
We could then accurately measure the weight percentage contributions of both solvents after we understood which peaks were associated with which solvents. The solvents seemed to be chemically identical once they discovered that there was no difference between those spectra. This indicated that there was likely a different cause for the performance issues.
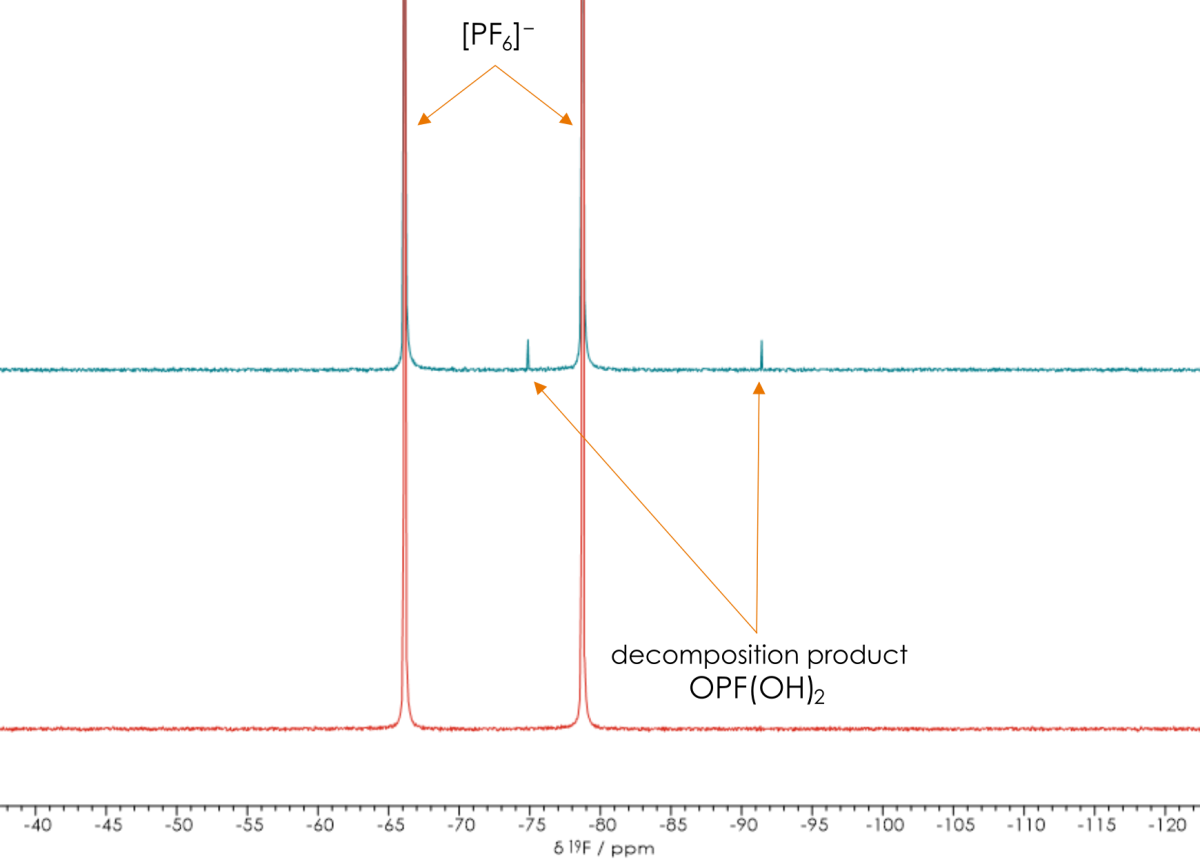
Following this, we strove to understand the electrolyte anion coming from the hexafluorophosphate lithium salt. To do this, we took a fluorine NMR spectrum. It was immediately evident that there was a difference in the spectrum. The coupling of the fluorines to the phosphorus in the hexafluorophosphate was causing a doublet on the coupled spectrum to arise.
At a different frequency, we also saw a different doublet. This was caused by a decomposition product, which suggested that the difference in performance was caused by a breakdown of the salt.Through our observations, we ascertained that lithium salt breaking down through a common hydrolysis reaction path was most likely the cause for the difference in performance.
Our analysis allowed the customer to address these electrolyte performance issues, which demonstrates how useful benchtop NMR can be in quality control applications.
How common a problem is decomposition when working with electrolytes in batteries, and how can benchtop NMR be used to address this?
There are many different routes through which common electrolytes such as carbonated solvents, tetraborate salts, and lithium hexafluorophosphate can decompose.The key to developing longer-life, safer batteries is characterizing those pathways, all of which can be monitored through the use of benchtop NMR. It is helpful to look at an example hydrolysis path to better understand this.By adding a single drop of water to a typical commercial electrolyte of hexafluorophosphate in dimethyl carbonate results in a hydrolysis reaction that includes hexafluorophosphate breaking down into a combination of pentafluorophosphate and lithium fluoride, with the former breaking down further to produce both fluorophosphoric acids and hydrofluoric acid. Phosphoric acid will be produced as well if sufficient water is present. We track reaction progress by taking spectra every 30 minutes. The reaction is fairly drastic and occurs quickly, even with just a relatively small amount of water.
We can observe the quantities of decomposition products forming and reaction rates through different stages of decomposition by recording and comparing this series of spectra. This ultimately allows better management and the ability to address these risks.
How can pulsed-field gradient NMR be used in the development of new liquid electrolytes?
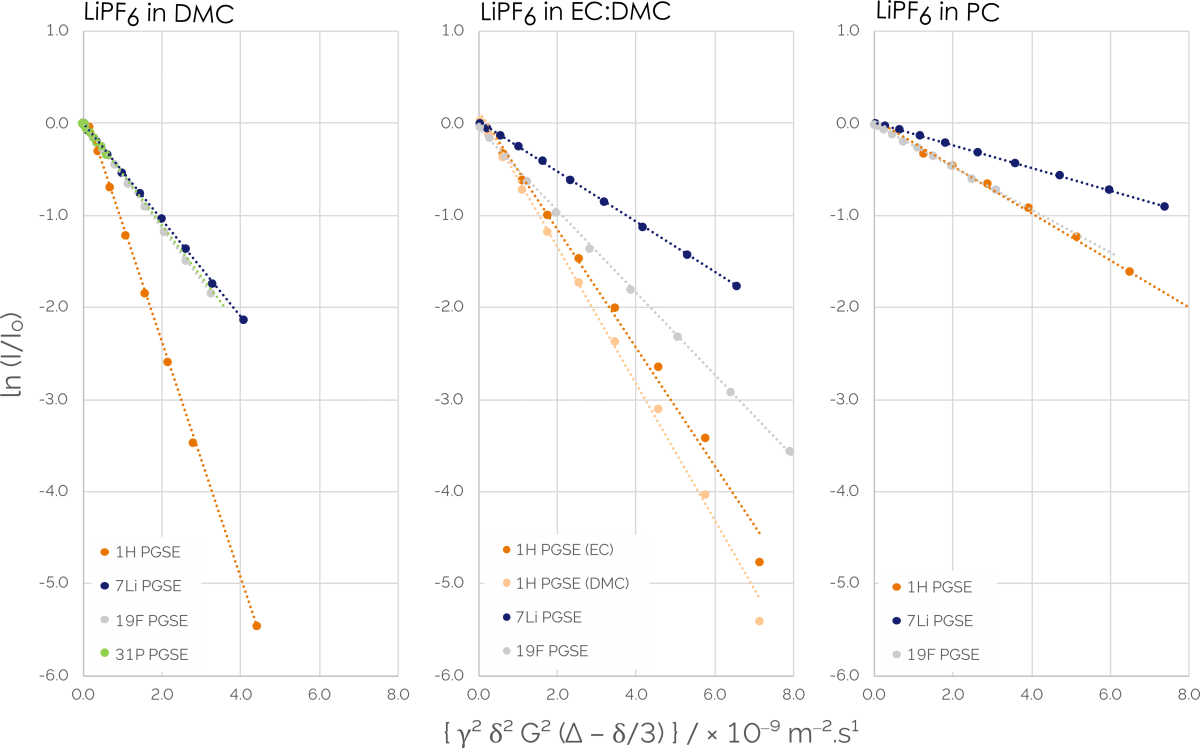
It can be the case that only viscosity and ionic conductivity and the use of these new electrolyte formulations are measured. It is recommended to measure the amount of charge carried by a particular ion species (also known as the transference number), as well as the diffusion coefficient of the different ion species. Doing so provides an improved prediction of the performance of an electrolyte when it is placed into a cell. We can use pulsed-field gradient spin echo NMR sequences to record all of these parameters, which tracks the change of signal amplitude proportional to the self-diffusion coefficient of a molecule.
We excite the sample with a typical 90-degree pulse and then apply a gradient pulse across that sample to prompt a change of phase across the sample to investigate this. If there was no diffusion, applying a second 180-degree pulse and an additional gradient pulse would allow us to see the same signal that we had previously.
It should be noted that we would see a decrease in the signal intensity dependent on the molecular diffusion coefficients and the strength of that gradient pulse and if molecules in our solution were to diffuse over the time between those two gradient pulses. We integrated the peaks in our spectra after taking a series of spectra at different gradient strengths, after which we should see a decrease in signal.
This data was fit to the Stejskal-Tanner equation so we could calculate the diffusion coefficient for the specific species in the solution. We could measure diffusion coefficients for the solvent molecules using hydrogen with a broadband NMR, as well as take measurements for each ionic species using phosphorus, boron, fluorine, or lithium.
We are then able to calculate the ionic conductivity of the cation transference and the electrolyte once we have those diffusion coefficients. These are highly useful parameters when developing new electrolytes. We can also understand how the breakdown process affects diffusion by using measurements on electrolytes at different stages of decomposition. This also allows us to better understand the physical properties of an electrolyte.
Finally, can you briefly summarize the reasons why benchtop NMR instruments like the X-Pulse are ideal for use with battery materials?
Benchtop NMR is perfect for the relatively simple spectra that we get from battery materials and vice versa. We can quantify key material concentrations in minutes, as well as take measurements of raw materials and their quality. This means we get almost instantaneous feedback on new formulations in R&D, ensuring that we understand key factors affecting performance and are making what we expected to make. We can unlock the physical properties of electrolytes through the use of diffusion experiments. When doing quality control, we can determine whether any decomposition is occurring or whether any contaminants are present by performing very quick measurements. Ultimately, the quality, performance, and cost of the final product are all improved by these experiments and investigations.
For More Information
James Sagar has been a Strategic Product and Applications Manager in benchtop NMR at Oxford Instruments since January 2019. He joined the company in 2015 as Product Manager for energy dispersive X-ray analysis, looking after the world’s first EDS detector for electron microscopes capable of detecting Li X-rays. Before this, James carried out post-doctoral research at University College London.