Products
Applications
Learning
The spread of illicit drugs has become a global problem, not only causing direct harm to people's physical and mental health, but also seriously affecting social and economic development. In addition to traditional drugs, many new psychoactive substances (NPS) have appeared in recent years, and they are becoming increasingly popular. New psychoactive substances are also known as "designer drugs" or "laboratory drugs". To avoid prosecution, criminals artificially design and modify the chemical structure of controlled drugs to obtain new types with effects similar to or even stronger than those of the controlled drugs. Due to the "new structure" of these substances, some traditional detection methods have become invalid, presenting great challenges to the drug prevention and control work of the whole society.
Nuclear Magnetic Resonance (NMR) technology can provide information on the connection of chemical molecular frameworks. It is the most direct tool for compound structure identification and can be used as an effective means to detect and identify new psychoactive substances. However, because traditional high-field superconducting NMR spectrometers have special requirements for operation and location, and because of the high cost of instrument use and maintenance, the application of NMR technology in rapid street drug screening is limited.
In response to the above problems, Oxford Instruments has provided a new benchtop NMR solution for rapid drug identification. Oxford Instruments’ X-Pulse benchtop NMR spectrometer uses rare earth permanent magnets, does not require liquid nitrogen, liquid helium or other refrigerants, is simple to operate and easy to maintain, and can quickly collect NMR spectra of suspicious drug samples.
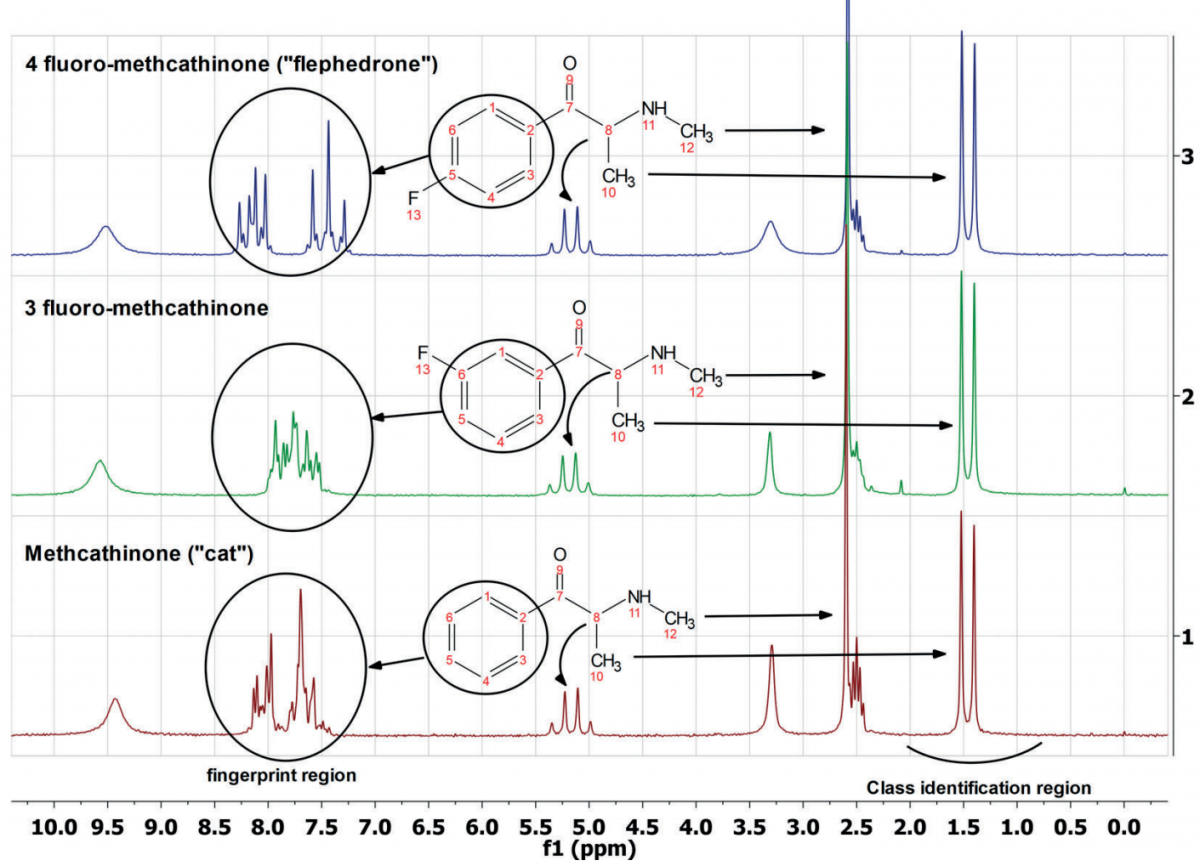
Figure 1. One-dimensional 1H spectra of three methcathinones
The supporting drug database enables rapid analysis and automatic comparison of the spectra of unknown samples against the spectra of known references. The samples can be tested on the X-Pulse after simple dissolution and processing, with the spectra collected automatically. Comparison and score matching is carried out against the built-in special drug database (which can be updated as new compounds are discovered) to give the best recommended match of possible substances. Even for a mixture of multiple drug substances, the instrument can automatically fit spectra against the drug fingerprint library and suggest possible combinations of mixed drugs, so that illicit drugs cannot be disguised. For most samples, fully automatic data collection, processing and analysis can usually be completed within 5-20 minutes.
Figure 1 shows the one-dimensional 1H spectrum of three methcathinones on a 60 MHz X-Pulse. The molecular structures of these three compounds are very similar, with only one fluorine atom or slight difference in the substitution position on the benzene ring. In the upfield "type identification area" of the chemical shift of the spectrum (for example, 0.5-2.0 ppm), the three have split double peaks, indicating that they belong to the same type of substance. In the downfield “fingerprint area” of the chemical shift of the spectrum (such as 7.0-9.0 ppm), the three show their own unique peak characteristics, thus permitting chemical structure differentiation. By pattern matching the "type identification area" and "fingerprint area" of the unknown substance with the database standard sample spectrum, the composition and structure of the unknown substance can be determined.
Amphetamine Analog
Take methcathinone as an example. Methcathinone is an analog of amphetamine and belongs to the strictly controlled Class I psychotropic drugs. It can cause acute health problems and permanent brain damage, and in severe cases can be life-threatening.
A batch of suspicious samples seized by public security agencies was analysed by X-Pulse and gas chromatography-mass spectrometry (GC-MS) at the same time. The results are shown in Table 1. The total number of samples analysed was 432, of which 13 (3.0%) could not be verified by NMR due to there being no GC-MS peaks, and 3 (0.7%) contained no active ingredients (API) or admixtures. Among the remaining 416 samples that were confirmed to contain API or admixtures, 387 (93.0%) of the NMR and GC-MS test results were matched exactly. On this basis, if the partially matched samples are added, the total number reached 412 (99.0%). Of equal importance, no false positives were found. This data demonstrates that X-Pulse can detect and identify street drugs with high accuracy and reliability.

| Sample Match Criteria | Quantity | Percentage |
| Total number of samples analysed | 432 | |
| Unable to verify (GC-MS does not produce peaks) x | 13 | 3.0% |
| No API or admixtures | 3 | 0.7% |
| Cannot match | 4 | 0.9% |
| Correct match (single component sample) | 374 | 86.6% |
| Correct match (two-component sample) | 13 | 3.0% |
| Partial matching (two-component or multi-component samples) | 25 | 5.8% |
| Verification of samples containing API or admixtures | 416 | |
| Exact match | 387 | 93.0% |
| Exact + partial match | 412 | 99.0% |
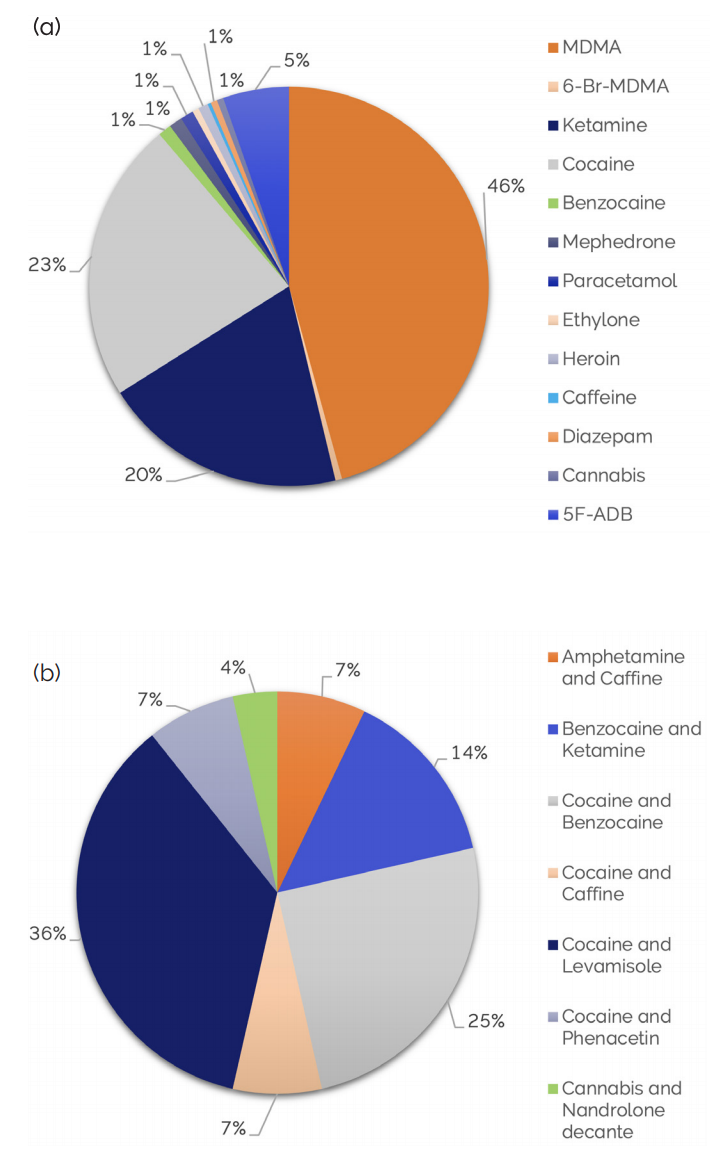
Figure 2. Types and quantities of street drugs detected by X-Pulse (a) One-component compound; (b) Two-component mixture [1]
Identifying street drugs - data for judicial enforcement
Through comparison and matching with the X-Pulse drug database, it was found that more than 400 questionable samples contained a variety of different drugs. Among street drugs consisting of single-component compounds, the most often detected were ecstasy (MDMA), cocaine and ketamine; in the two-component mixture samples, cocaine/levamisole, cocaine/benzocaine, and other admixtures were mainly found (Figure 2).
The results can provide valuable references for public security judicial identification agencies to understand the types and prevalence of local drugs, and to formulate corresponding control strategies and action plans.
References:
[1] Antonides LH et al., Rapid Identification of Novel Psychoactive and Other Controlled Substances Using Low-Field 1H NMR Spectroscopy, ACS Omega. 2019, 4: 7103-7112
[2] Mewis R et al., Quantification of MDMA in seized tablets using benchtop 1H NMR spectroscopy in the absence of internal standards, Forensic Chemistry, 2020, 20: 100263.