Products
Applications
Learning
Solid state batteries that deliver the energy and power density, as well as cell cycle lifetimes required for future generations of rechargeable batteries are a core research focus both in academia and industry. In Li-ion batteries, the lithium ions have to be mobile in order to be effective charge carriers. This mobility means they are slightly more “liquid-like” and therefore benchtop NMR spectroscopy can provide information about the mobility of the lithium which determines the electrolyte conductivity. This information can be accessed using an X-Pulse NMR spectrometer using pulsed-field gradients and a pulsed-field gradient stimulated echo sequence (PFGSTE) directly measures the diffusion coefficient of the lithium ions.
The materials being considered for this application range from polymers to ceramics with a diverse span of expected lithium diffusion coefficients and conductivities. One example is argyrodites which are sulfide-based lithium-ion superconductors with high ionic conductivity at room temperature. These are currently being commercialised by companies including BASF. We have recently measured lithium diffusion in these compounds using the X-Pulse benchtop NMR, with very good agreement with high-field solid state NMR. Using both techniques diffusion coefficients of (5.9±0.7)×10−12 m2.s−1 were obtained.
Talk to our experts
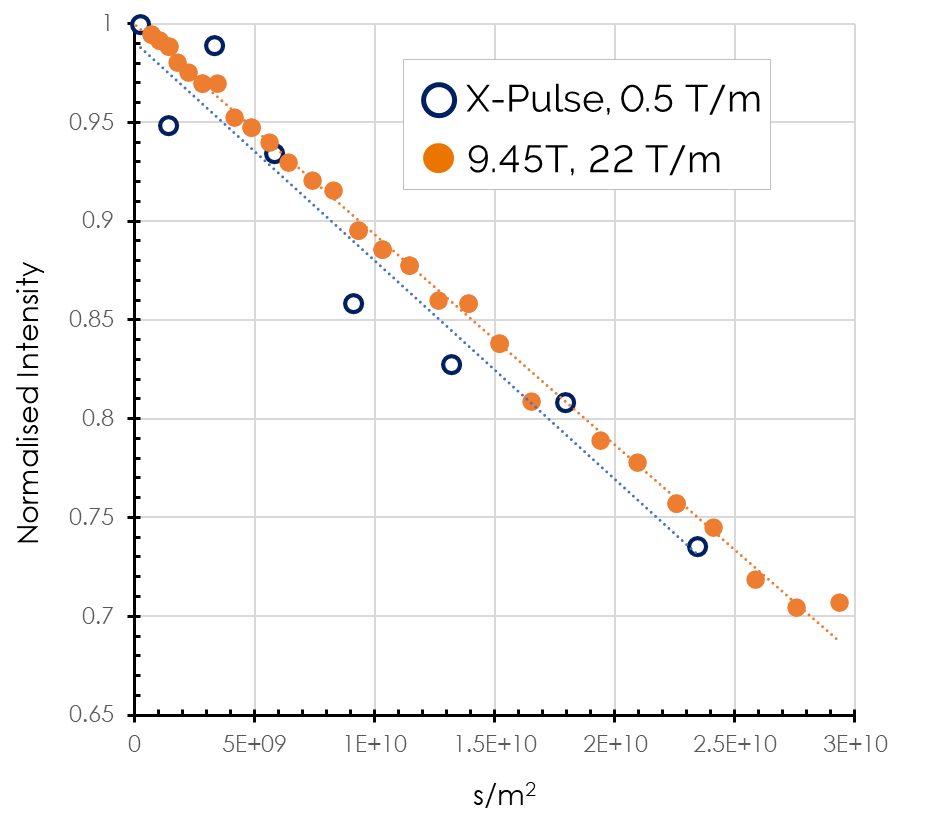
Comparison of argyrodite electrolyte diffusion coefficients measured by both benchtop X-Pulse NMR spectrometer (PFGSTE sequence) and solid-state NMR.
This is almost at the limit of what is possible with current benchtop NMR spectrometers. However, if the diffusion data only is sufficient for characterisation and an NMR spectrum is not required, we can investigate using time-domain benchtop NMR (TD-NMR) techniques which achieve significantly higher pulsed field gradients. The table below summarises the benchtop NMR electrolyte characterisation capability for measurement of diffusion coefficients and evaluates both spectroscopy and time domain methods for some example electrolyte materials with differing diffusivities.
| Electrolyte Material | Diffusion Coefficient | Benchtop NMR spectroscopy | TD-NMR |
| Polymer salt mixtures with high diffusion coefficients | 10⁻⁷ | Yes | Yes |
| Pure polymer electrolytes. E.g. LiPON - lithium phosphorus oxynitride | 10⁻¹¹ | Yes | Yes |
| Argyrodites, typical nominal composition of Li₆PS₅Cl | 10⁻¹² | Maybe | Yes |
| Perovskite, ceramic or garnet electrolytes. E.g., LLZTO - lithium lanthanum zirconium titanium oxide | 10⁻¹⁸ | No | Maybe |
We would like to thank Dr Gregory Rees and colleagues from the Sir Peter Bruce Group at the Department of Materials, University of Oxford whose contributions and collaboration were integral to the successful development of this application.