Products
Applications
Learning
5th March 2024 | Author: Sheida Rajabi
The X-Pulse with a fully tuneable broadband (X-)channel has been proven to be capable of observing many NMR active nuclei over a broad range of frequencies. So far, the nuclei shown in dark blue in the periodic table have been measured on the X-Pulse with the convenience and ease of use of a benchtop system. When it comes to Bromine, solid-state NMR is primarily used in research due to the reason that both bromine isotopes (79Br and 81Br) are quadrupolar (S= 3/2) that yield broad signals, which sometimes are hardly detectable with high-resolution spectrometers, even in the small molecules. However, both nuclei have an excellent natural abundance of around 50% and resonance frequency quite similar to 13C (Frequency of 13C @1.4T =15.01 MHz). Some properties of both bromine isotopes are listed in table 1. Given this information, we aimed to run Br NMR on our 60 MHz X-Pulse system, using a 3 mol/l solution of KBr in D2O. Both isotopes result in a resonance at a chemical shift (δ) of 8.57 ppm and a linewidth (ν½) of 539 and 395 Hz for 79Br and 81Br, respectively. As shown in Figure 1, 81Br appeared slightly narrower than 79Br which can make it a nucleus of interest in solution NMR. Therefore, we can add 79Br/81Br (in orange colour in the periodic table) to the list of nuclei that have been demonstrated to be observable on the X-Pulse.
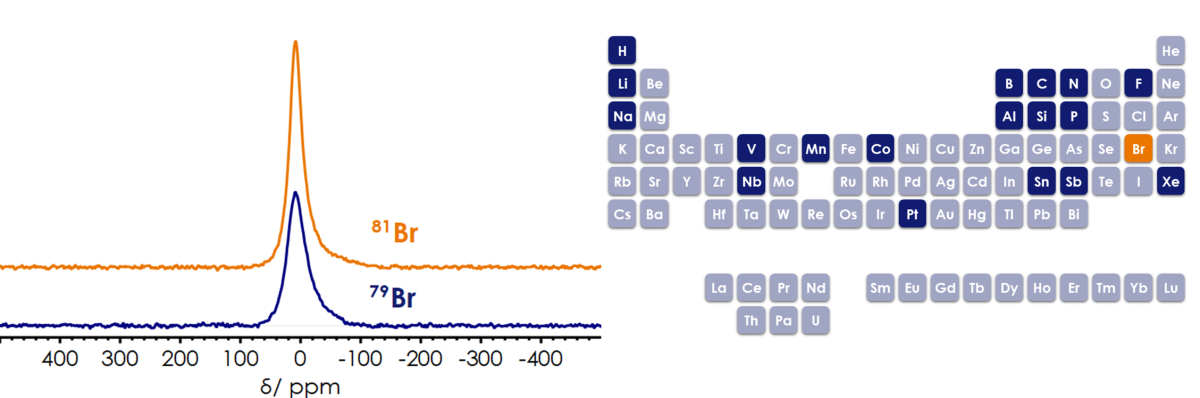
Figure 1. 1D bromine NMR of KBr dissolved in D2O measured on the X-Pulse. (number of scans: 512, total experiment time: 20 minutes (left). Nuclei observed on the X-Pulse in dark blue (right).
| Isotope | Nuclear Spin | Natural Abundance (%) | Larmor Frequency @ 1.4T (MHz) | Relative Receptivity versus ¹³C |
| ⁷⁹Br | 3/2 | 50.69 | 14.99 | 237 |
| ⁸¹Br | 3/2 | 49.31 | 16.16 | 288 |
Table 1. NMR properties of active nuclei 79Br and 81Br.
Next, the spin-lattice (or longitudinal) relaxation time (T1) and spin-spin (or transverse) relaxation time (T2) of both bromine isotopes were obtained using Inversion Recovery (InvRec) and spin-echo (CPMG) pulse sequences, respectively, revealing very short relaxation time for both nuclei, as summarised in Table 2. For the calculation of T1, an array of InvRec experiments were performed while varying the recovery time parameter ranging from 1 - 640 ms, followed by fitting the signal integrated area to an exponential equation shown in Figure 2. After having T1 optimized, we can safely run quantitative bromine measurements on the X-Pulse. T2 relaxation time was also obtained by collecting an array of CPMG experiments keeping a constant echo time (τ) while varying the number of echoes. The resulting attenuation of the signals is fitted to an exponential equation depicted in Figure 3, to obtain T2.
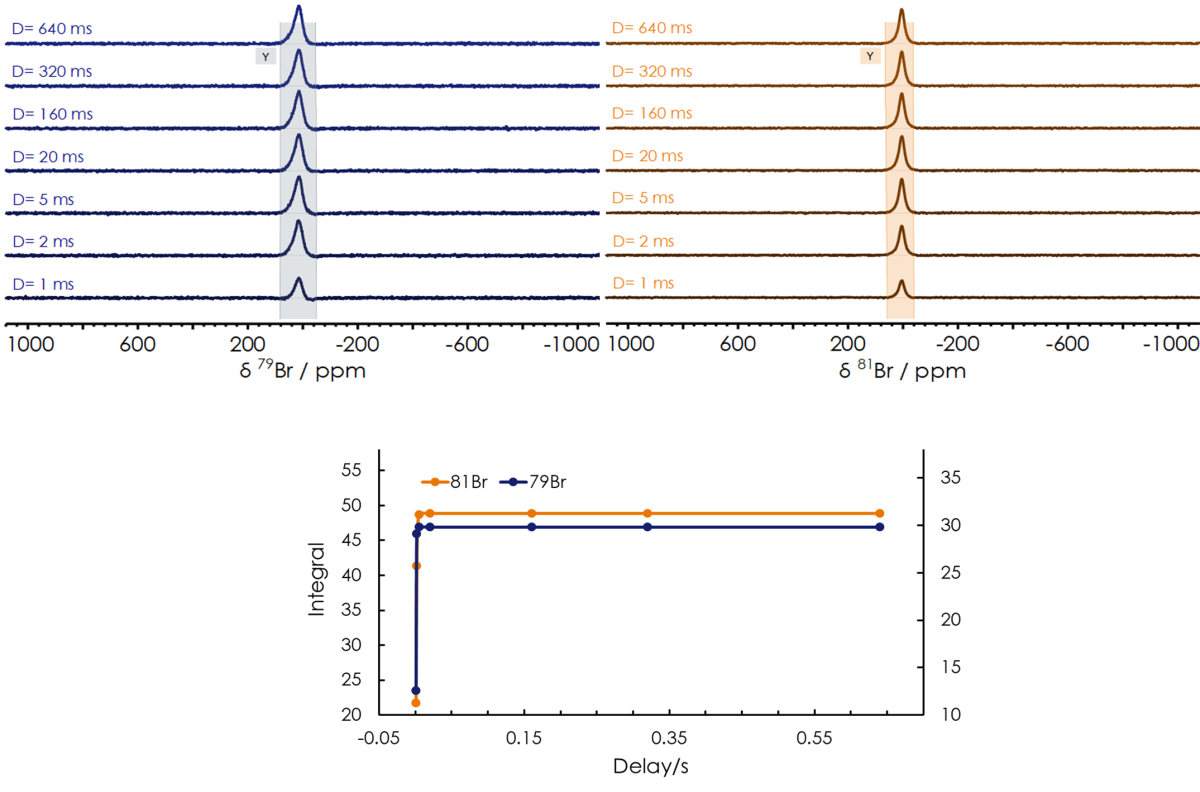
Figure 2. Inversion recovery experiment for the measurement of T1 in a 3 mol/l solution of KBr in D2O.
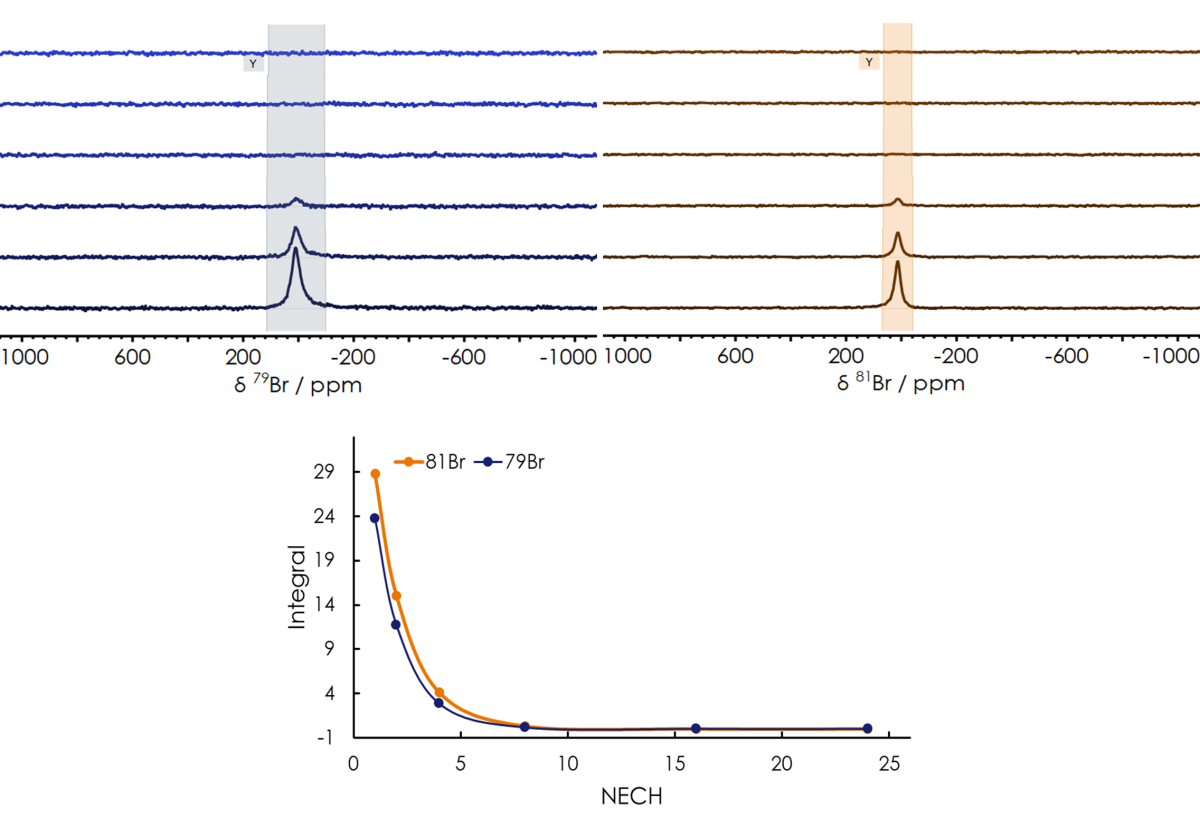
Figure 3. CPMG experiment for the measurement of T2 in a 3 mol/l solution of KBr in D2O. Each echo has a duration of 2×τ.
| Isotope | T₁(ms) | T₂(ms) |
| ⁷⁹Br | 0.3 | 0.6 |
| ⁸¹Br | 0.8 | 0.7 |
Table 2. T1 and T2 of 79Br and 81Br.
In this blog, we demonstrate the capability of the X-Pulse broadband benchtop NMR spectrometer for the measurement of bromine nuclei in the most convenient way. Switching between different nuclei is very rapid, and only requires the selection of the nucleus of interest in the software and subsequently optimising the probe to the right frequency. Combining all the features found on the X-pulse, we can consider it as great potential for the functionality of benchtops in both academic and industrial environments.
Sheida Rajabi,
Applications Scientist, Oxford Instruments
Sheida is an Application Scientist based out of Wiesbaden, Germany. She got her PhD in inorganic chemistry from the Georg-August Göttingen University where she worked on the synthesis and characterisation of molecular ruthenium-based complexes and their application as water oxidation catalysts.